Abstract
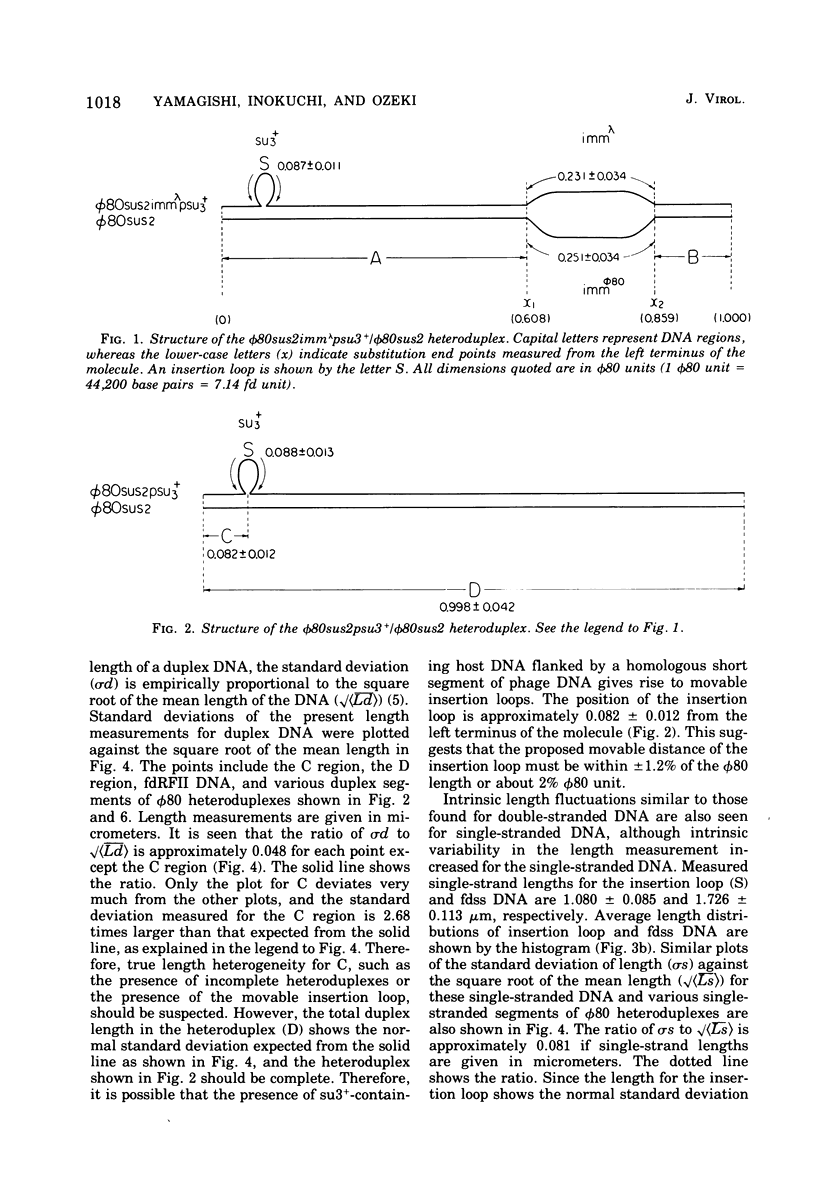
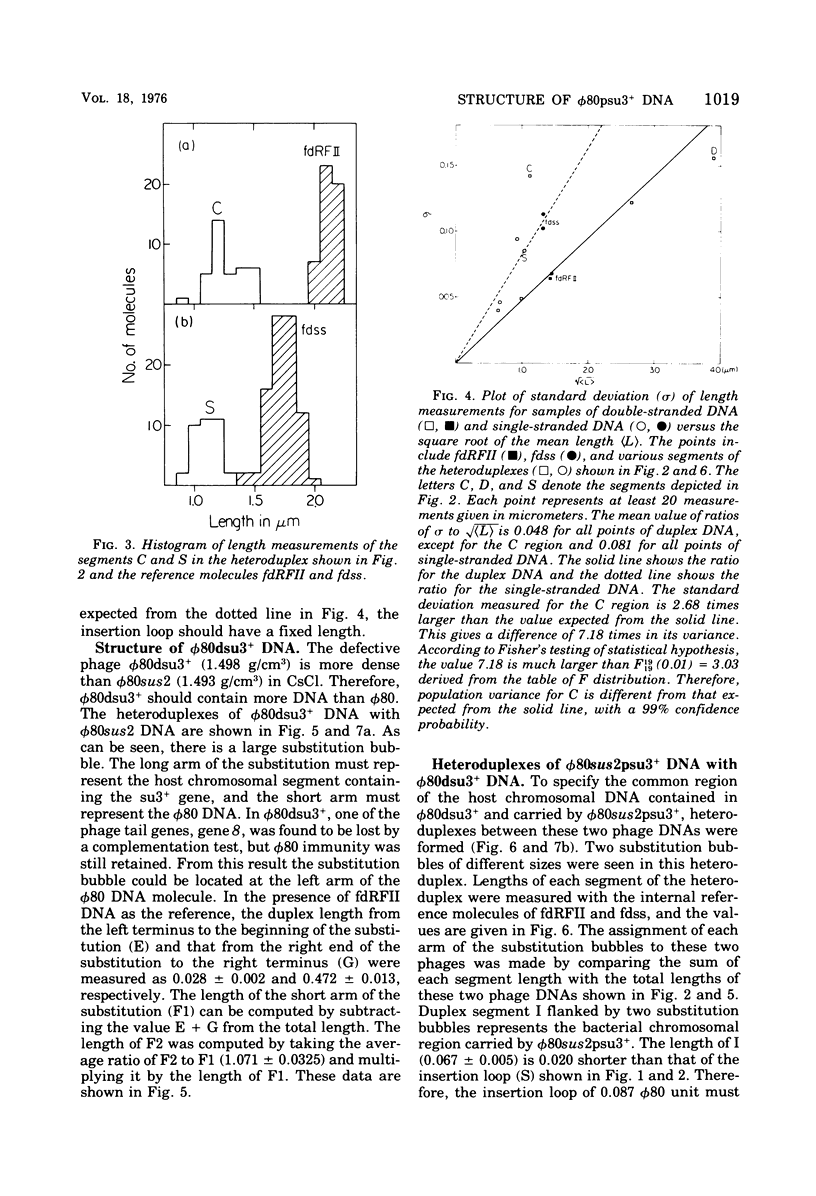
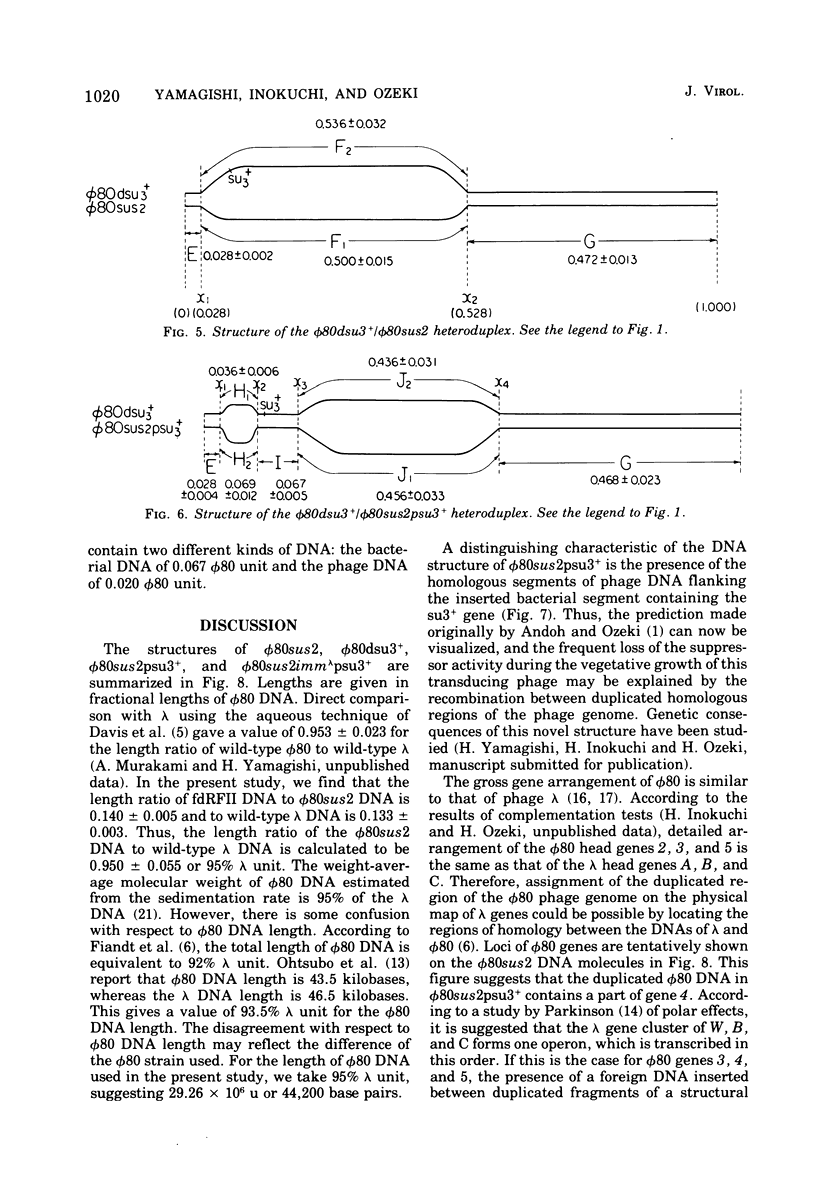
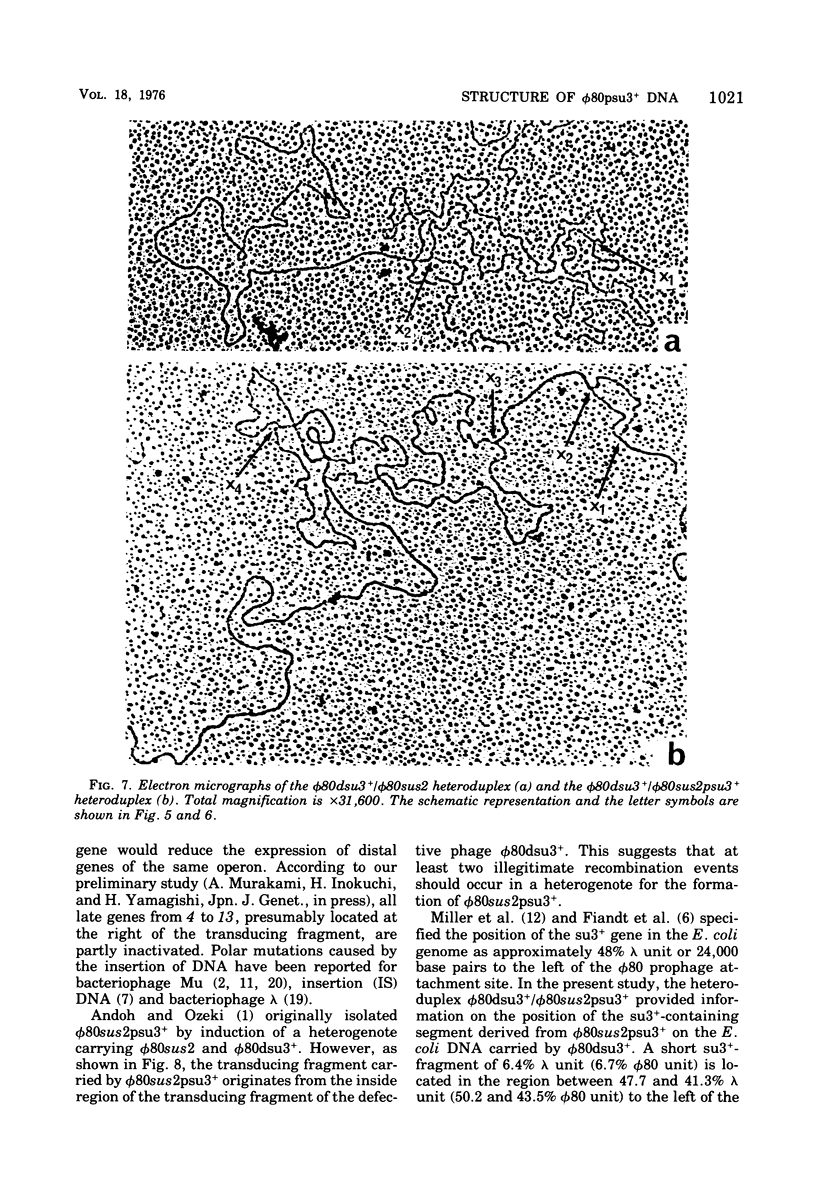
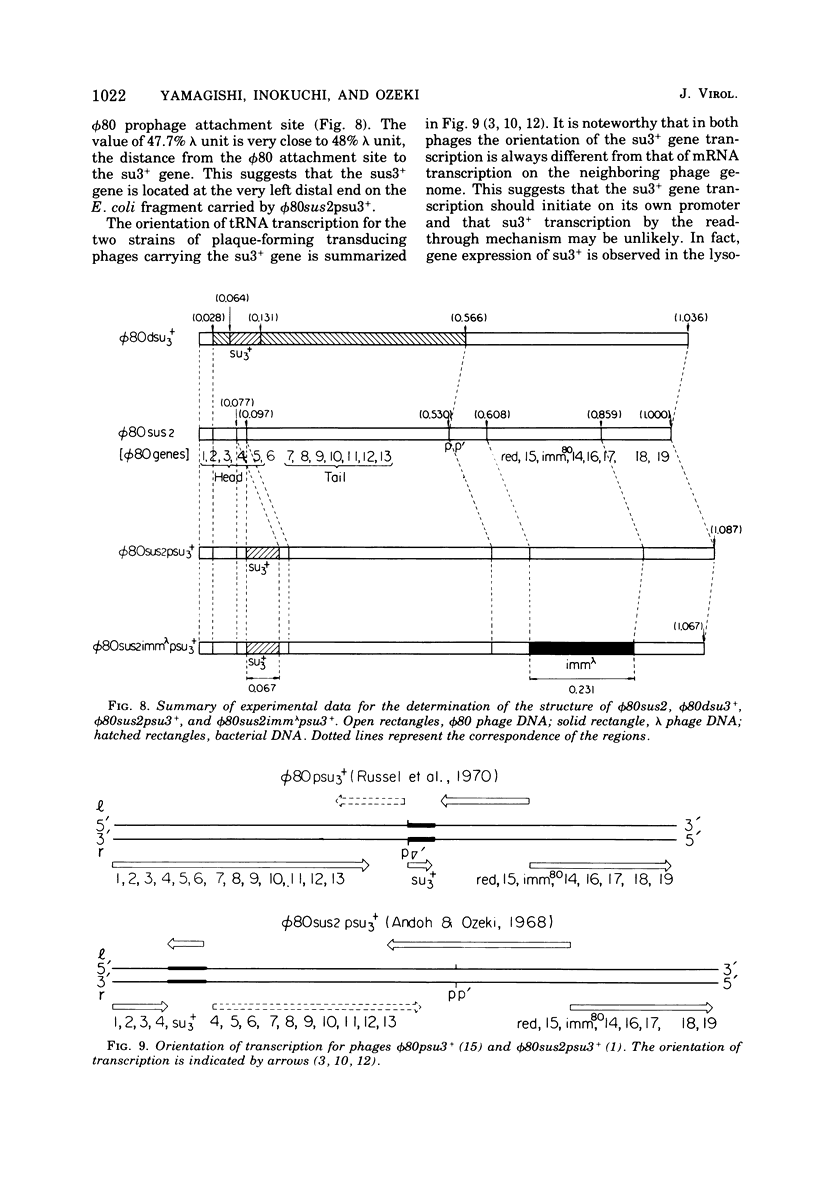
DNA molecules of phi 80sus2psu3+ and phi 80dsu3+ isolated by Andoh and Ozeki (1968) were studied by the electron microscope heteroduplex method. The phi 80sus2psu3+ and phi 80dsu3+ DNA lengths were found to be 108.7 and 103.3% of the phi 80 DNA, respectively. The phi 80sus2psu3+/phi 80 heteroduplex shows an insertion loop of 8.7% of the phi 80 DNA which migrates from 7.7 to 9.7%, as measured relative to the left (0%) and right (100%) termini of the mature phi 80 DNA molecule. The region of loop migration occupies the central region of the phi 80 head gene cluster. The presence of su3+-containing Escherichia coli DNA of 6.7% phi 80 unit flanked by two homologous regions of phage DNA of 2.0% of phi 80 unit gives rise to a movable insertion loop. In phi 80dsu3+, from which phi 80sus2psu3+ was derived, 50.5% of the phi 80 DNA at the left arm was replaced by E. coli DNA containing the su3+ gene, equivalent to about 53.8% phi 80 unit in length. The phi 80sus2psu3+/phi 80dsu3+ heteroduplex appears as a double-stranded molecule that bifurcates into two clearly visible single-stranded regions, rejoins, bifurcates, and rejoins again. The middle double-stranded stretches of 6.7% phi 80 unit correspond to the E. coli DNA inserted in phi 80sus2psu3+. Therefore the transducing fragment carried by phi 80sus2psu3+ originates from the inside region of the transducing fragment of defective phage phi 80dsu3+ by at least two illegitimate recombination events.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andoh T., Ozeki H. Suppressor gene Su3+ of E. coli, a structural gene for tyrosine TRNA. Proc Natl Acad Sci U S A. 1968 Mar;59(3):792–799. doi: 10.1073/pnas.59.3.792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boram W., Abelson J. Bacteriophage Mu integration: on the mechanism of Mu-induced mutations. J Mol Biol. 1971 Nov 28;62(1):171–178. doi: 10.1016/0022-2836(71)90137-9. [DOI] [PubMed] [Google Scholar]
- Daniel V., Beckmann J. S., Sarid S., Grimberg J. I., Herzberg M., Littauer U. Z. Purification and in vitro transcription of a transfer RNA gene. Proc Natl Acad Sci U S A. 1971 Sep;68(9):2268–2272. doi: 10.1073/pnas.68.9.2268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirsch H. J., Saedler H., Starlinger P. Insertion mutations in the control region of the galactose operon of E. coli. II. Physical characterization of the mutations. Mol Gen Genet. 1972;115(3):266–276. doi: 10.1007/BF00268890. [DOI] [PubMed] [Google Scholar]
- KELLENBERGER G., ZICHICHI M. L., WEIGLE J. J. Exchange of DNA in the recombination of bacteriophage lambda. Proc Natl Acad Sci U S A. 1961 Jun 15;47:869–878. doi: 10.1073/pnas.47.6.869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lozeron H. A., Szybalski W. Congruent transcriptional controls and heterology of base sequences in coliphages lambda and phi-80. Virology. 1969 Nov;39(3):373–388. doi: 10.1016/0042-6822(69)90085-3. [DOI] [PubMed] [Google Scholar]
- Martuscelli J., Taylor A. L., Cummings D. J., Chapman V. A., DeLong S. S., Cañedo L. Electron microscopic evidence for linear insertion of bacteriophage MU-1 in lysogenic bacteria. J Virol. 1971 Oct;8(4):551–563. doi: 10.1128/jvi.8.4.551-563.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller R. C., Jr, Besmer P., Khorana H. G., Fiandt M., Szybalski W. Studies on polynucleotides. XCVII. Opposing orientations and location of the su+3 gene in the transducing coliphages phi-80psu+3 and phi-80dsu+3su-3. J Mol Biol. 1971 Mar 14;56(2):363–368. doi: 10.1016/0022-2836(71)90470-0. [DOI] [PubMed] [Google Scholar]
- Otsubo E., Lee H. J., Deonier R. C., Davidson N. Electron microscope heteroduplex studies of sequence relations among plasmids of Escherichia coli. VI. Mapping of F14 sequences homologous to phi 80dmetBJF and phi 80dargECBH bacteriophages. J Mol Biol. 1974 Nov 15;89(4):599–618. doi: 10.1016/0022-2836(74)90038-2. [DOI] [PubMed] [Google Scholar]
- Parkinson J. S. Genetics of the left arm of the chromosome of bacteriophage lambda. Genetics. 1968 Jul;59(3):311–325. doi: 10.1093/genetics/59.3.311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell R. L., Abelson J. N., Landy A., Gefter M. L., Brenner S., Smith J. D. Duplicate genes for tyrosine transfer RNA in Escherichia coli. J Mol Biol. 1970 Jan 14;47(1):1–13. doi: 10.1016/0022-2836(70)90397-9. [DOI] [PubMed] [Google Scholar]
- Sato K. Genetic map of bacteriophage phi80: genes on the right arm. Virology. 1970 Apr;40(4):1067–1069. doi: 10.1016/0042-6822(70)90156-x. [DOI] [PubMed] [Google Scholar]
- Sato K., Nishimune Y., Sato M., Numich R., Matsushiro A. Suppressor-sensitive mutants of coliphage phi-80. Virology. 1968 Apr;34(4):637–649. [PubMed] [Google Scholar]
- Sharp P. A., Hsu M. T., Otsubo E., Davidson N. Electron microscope heteroduplex studies of sequence relations among plasmids of Escherichia coli. I. Structure of F-prime factors. J Mol Biol. 1972 Nov 14;71(2):471–497. doi: 10.1016/0022-2836(72)90363-4. [DOI] [PubMed] [Google Scholar]
- Shimada K., Weisberg R. A., Gottesman M. E. Prophage lambda at unusual chromosomal locations. II. Mutations induced by bacteriophage lambda in Escherichia coli K12. J Mol Biol. 1973 Oct 25;80(2):297–314. doi: 10.1016/0022-2836(73)90174-5. [DOI] [PubMed] [Google Scholar]
- TAYLOR A. L. BACTERIOPHAGE-INDUCED MUTATION IN ESCHERICHIA COLI. Proc Natl Acad Sci U S A. 1963 Dec;50:1043–1051. doi: 10.1073/pnas.50.6.1043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamagishi H., Nakamura K., Ozeki H. Cohesion occurring between DNA molecules of temperate phages phi 80 and lambda or phi 81. Biochem Biophys Res Commun. 1965 Sep 22;20(6):727–732. doi: 10.1016/0006-291x(65)90077-x. [DOI] [PubMed] [Google Scholar]
- Yamagishi H., Ozeki H. Comparative study of thermal inactivation of phage phi 80 and lambda. Virology. 1972 May;48(2):316–322. doi: 10.1016/0042-6822(72)90042-6. [DOI] [PubMed] [Google Scholar]



