Abstract
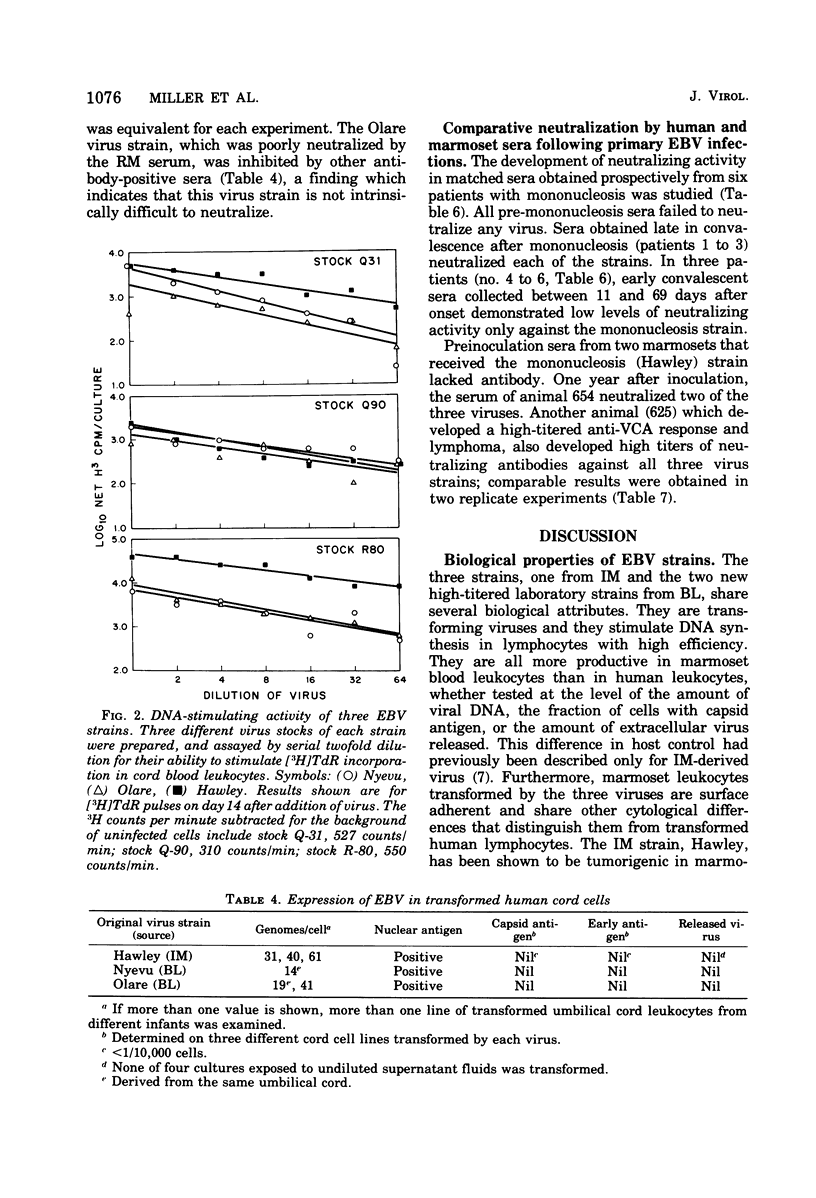
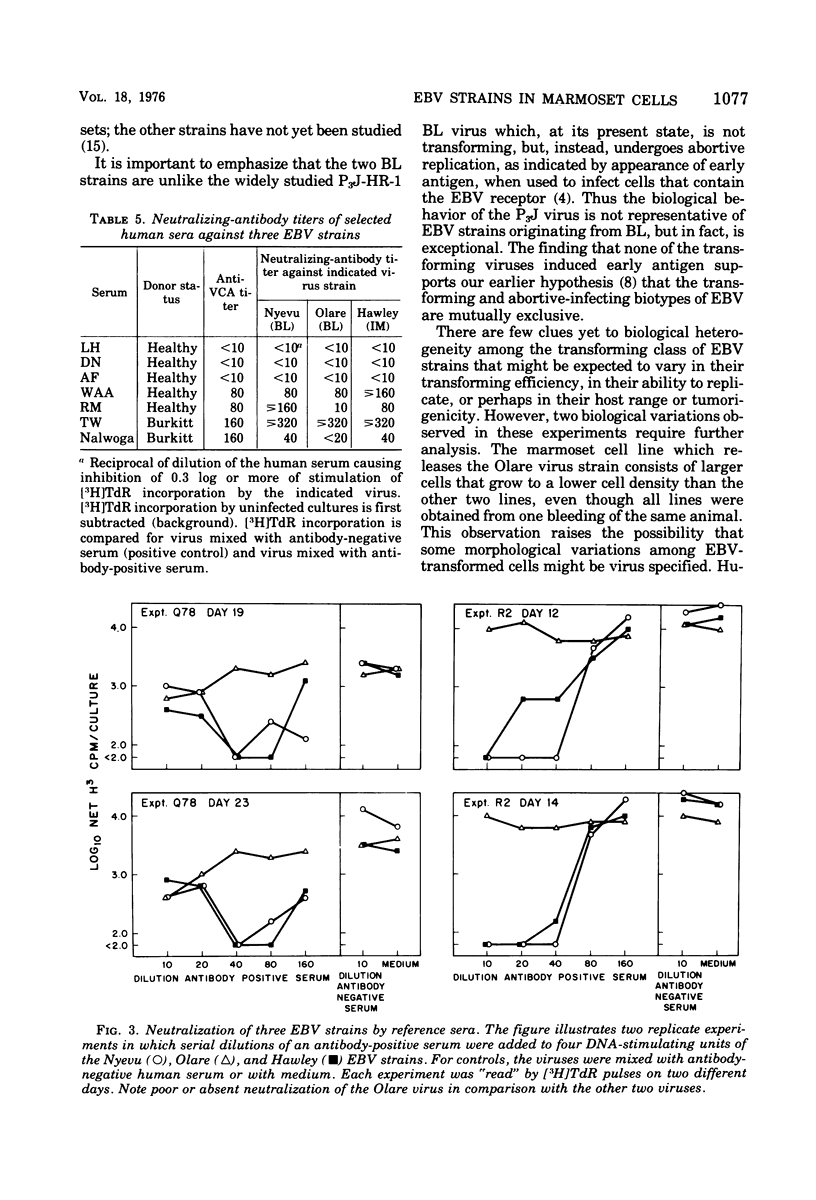
Three strains of Epstein-Barr virus (EBV), two from Burkitt lymphoma (BL) and one from infectious mononucleosis (IM) were used to transform separate cultures of the same batch of primary marmoset leukocytes, and the viruses released from the transformants were compared. The three viruses shared properties of the transforming biotype of EBV, namely, stimulation of DNA synthesis and immortalization of cord blood leukocytes, and failure to induce "early antigen" in lymphoblast lines. All viruses produced more virus in transformed marmoset cells than in transformed human cells, as measured by the number of EBV genomes detected by complementary RNA/DNA hybridization, by virus capsid antigen expression, or by released virions and biologically active virus. Reference human sera and sera from primary EBV infections were used to compare the three virus strains in a virus neutralization test based on inhibition of stimulation of DNA synthesis. Specimens taken late in convalescence from patients with mononucleosis and sera from marmosets experimentally infected with virus from a patient with mononucleosis neutralized the homologous virus, as well as the two virus strains isolated from patients with BL. This finding indicates that viral antigens that elicit neutralizing antibodies are shared among the strains. However, in certain sera the neutralizing-antibody titer against one strain was consistently higher than against another strain. Furthermore, sera taken early after onset of IM contained low levels of neutralizing antibody against IM-derived virus, but failed to neutralize BL-derived virus. These latter findings suggest the existence of heterogeneity among surface antigens of EBVs. The results emphasize the biological and antigenic similarity of EBV isolates from BL and IM and do not suggest major subtype variations. It remains to be determined whether antigenic diversity such as described or virus genome variation detectable by other means is epidemiologically significant.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams A. Concentration of Epstein-Barr virus from cell culture fluids with polyethylene glycol. J Gen Virol. 1973 Sep;20(3):391–394. doi: 10.1099/0022-1317-20-3-391. [DOI] [PubMed] [Google Scholar]
- Chang R. S. Neutralizing activity in human sera against the leukocyte-transforming agent. J Infect Dis. 1973 Jul;128(1):50–55. doi: 10.1093/infdis/128.1.50. [DOI] [PubMed] [Google Scholar]
- Henle G., Henle W. Immunofluorescence in cells derived from Burkitt's lymphoma. J Bacteriol. 1966 Mar;91(3):1248–1256. doi: 10.1128/jb.91.3.1248-1256.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henle W., Henle G., Zajac B. A., Pearson G., Waubke R., Scriba M. Differential reactivity of human serums with early antigens induced by Epstein-Barr virus. Science. 1970 Jul 10;169(3941):188–190. doi: 10.1126/science.169.3941.188. [DOI] [PubMed] [Google Scholar]
- Katsuki T., Hinuma Y. Characteristics of cell lines derived from human leukocytes transformed by different strains of Epstein-Barr virus. Int J Cancer. 1975 Feb 15;15(2):203–210. doi: 10.1002/ijc.2910150205. [DOI] [PubMed] [Google Scholar]
- Klein G., Sugden B., Leibold W., Menezes J. Infection of EBV-genome-negative and -positive human lymphoblastoid cell lines with biologically different preparations of EBV. Intervirology. 1974;3(4):232–244. doi: 10.1159/000149760. [DOI] [PubMed] [Google Scholar]
- Miller G., Lipman M. Release of infectious Epstein-Barr virus by transformed marmoset leukocytes. Proc Natl Acad Sci U S A. 1973 Jan;70(1):190–194. doi: 10.1073/pnas.70.1.190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller G., Robinson J., Heston L. Immortalizing and nonimmortalizing laboratory strains of Epstein-Barr Virus. Cold Spring Harb Symp Quant Biol. 1975;39(Pt 2):773–781. doi: 10.1101/sqb.1974.039.01.089. [DOI] [PubMed] [Google Scholar]
- Miller G., Shope T., Lisco H., Stitt D., Lipman M. Epstein-Barr virus: transformation, cytopathic changes, and viral antigens in squirrel monkey and marmoset leukocytes. Proc Natl Acad Sci U S A. 1972 Feb;69(2):383–387. doi: 10.1073/pnas.69.2.383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monroe J. H., Brandt P. M. Rapid semiquantitative method for screening large numbers of virus samples by negative staining electron microscopy. Appl Microbiol. 1970 Aug;20(2):259–262. doi: 10.1128/am.20.2.259-262.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nonoyama M., Pagano J. S. Detection of Epstein-Barr viral genome in nonproductive cells. Nat New Biol. 1971 Sep 22;233(38):103–106. doi: 10.1038/newbio233103a0. [DOI] [PubMed] [Google Scholar]
- Pagano J. S. The Epstein-Barr Virus and malignancy: molecular evidence. Cold Spring Harb Symp Quant Biol. 1975;39(Pt 2):797–805. doi: 10.1101/sqb.1974.039.01.092. [DOI] [PubMed] [Google Scholar]
- Pritchett R. F., Hayward S. D., Kieff E. D. DNA of Epstein-Barr virus. I. Comparative studies of the DNA of Epstein-Barr virus from HR-1 and B95-8 cells: size, structure, and relatedness. J Virol. 1975 Mar;15(3):556–559. doi: 10.1128/jvi.15.3.556-559.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson J. Assay for Epstein-Barr virus based on stimulation of DNA synthesis in mixed leukocytes from human umbilical cord blood. J Virol. 1975 May;15(5):1065–1072. doi: 10.1128/jvi.15.5.1065-1072.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shope T., Dechairo D., Miller G. Malignant lymphoma in cottontop marmosets after inoculation with Epstein-Barr virus. Proc Natl Acad Sci U S A. 1973 Sep;70(9):2487–2491. doi: 10.1073/pnas.70.9.2487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silvestre D., Kourilsky F. M., Klein G., Yata Y., Neauport-Sautes C., Levy J. P. Relationship between the EBV-associated membrane antigen on Burkitt lymphoma cells and the viral envelope, demonstrated by immunoferritin labelling. Int J Cancer. 1971 Sep 15;8(2):222–233. doi: 10.1002/ijc.2910080206. [DOI] [PubMed] [Google Scholar]
- Sim C., Watson D. H. The role of type specific and cross reacting structural antigens in the neutralization of herpes simplex virus types 1 and 2. J Gen Virol. 1973 May;19(2):217–233. doi: 10.1099/0022-1317-19-2-217. [DOI] [PubMed] [Google Scholar]
- Svedmyr A., Demissie A., Klein G., Clifford P. Antibody patterns in different human sera against intracellular and membrane-antigen complexes associated with Epstein-Barr virus. J Natl Cancer Inst. 1970 Mar;44(3):595–610. [PubMed] [Google Scholar]