Abstract
Stocks of cloned helper-independent Rous sarcoma virus (RSV) spontaneously segregate transformation-defective (td) mutants that appear to have an RNA genome composed of smaller subunits than those of the patent virus. Differential hybridization and competitive hybridization techniques involving reactions between viral RNA and proviral sequences in host cell DNA (under conditions of initial DNA excess) were used to measure the extent of the deletion in a td mutant of Prague strain (Pr) of RSV (Pr RSV-C). Viral 60 to 70S RNA sequences labeled to 1 to 5 × 107 counts per min per μg with 125I were characterized with respect to their properties in hybridization reactions and used to reinforce data obtained with [3H]RNA of lower specific activity. By these techniques, about 13% ± 3% of the sequences Pr RSV-C that formed hybrids with DNA from virus-induced sarcomas appeared to be deleted from the genome of td Pr RSV-C. Studies comparing hybridization of RNA from Pr RSV-C and td Pr RSV-C with RSV-related sequences in normal cells, and competition experiments with RNA from the endogenous chicken oncornavirus Rous-associated virus type 0 (RAV-0) provided evidence that the majority, if not all, of the RNA sequences of Pr RSV-C deleted from its transformation-defective mutant are not represented in normal chicken DNA. Competition studies with a leukosis virus, RAV-7, indicated this virus also lacks a genome segment of about the same size as the deletion in the td mutant. Finally, the genome of all three “exogenous” viruses was found to lack a small segment (about 12%) of sequences present in the endogenous provirus of RAV-O.
Full text
PDF

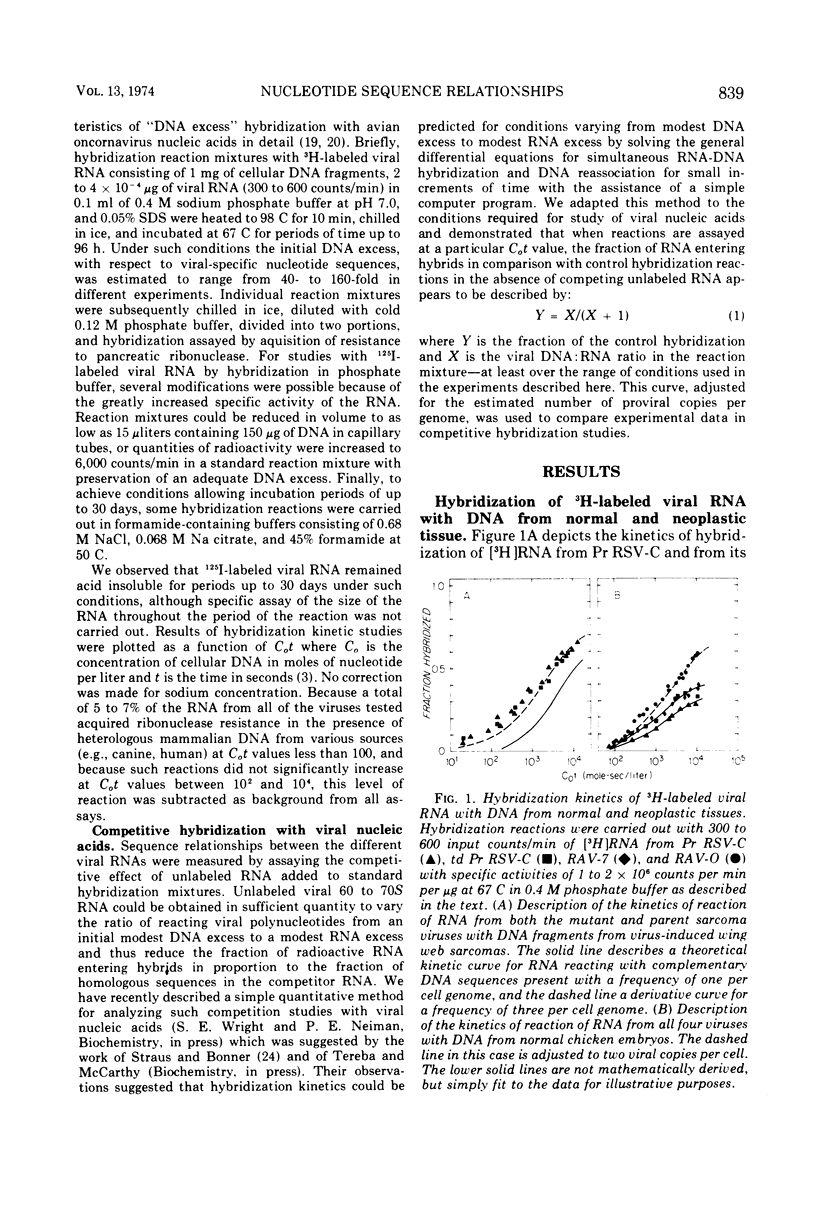

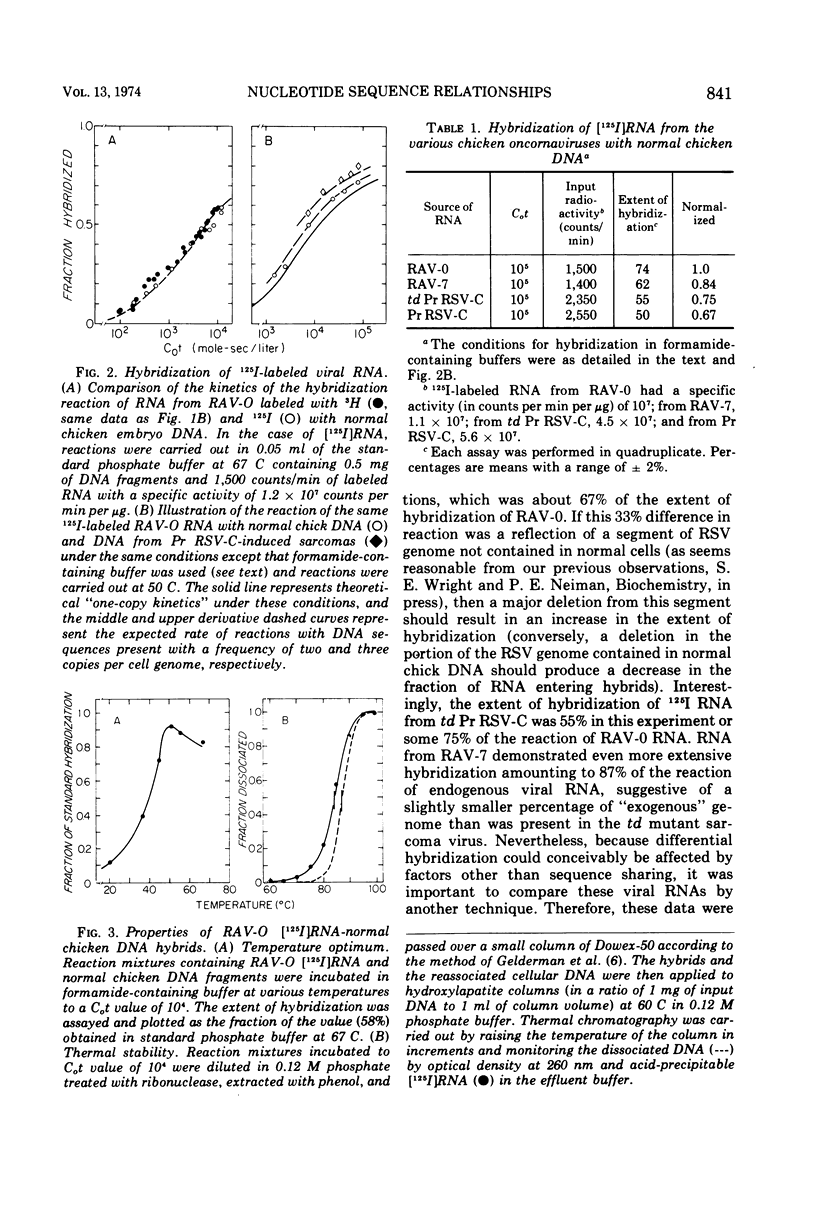
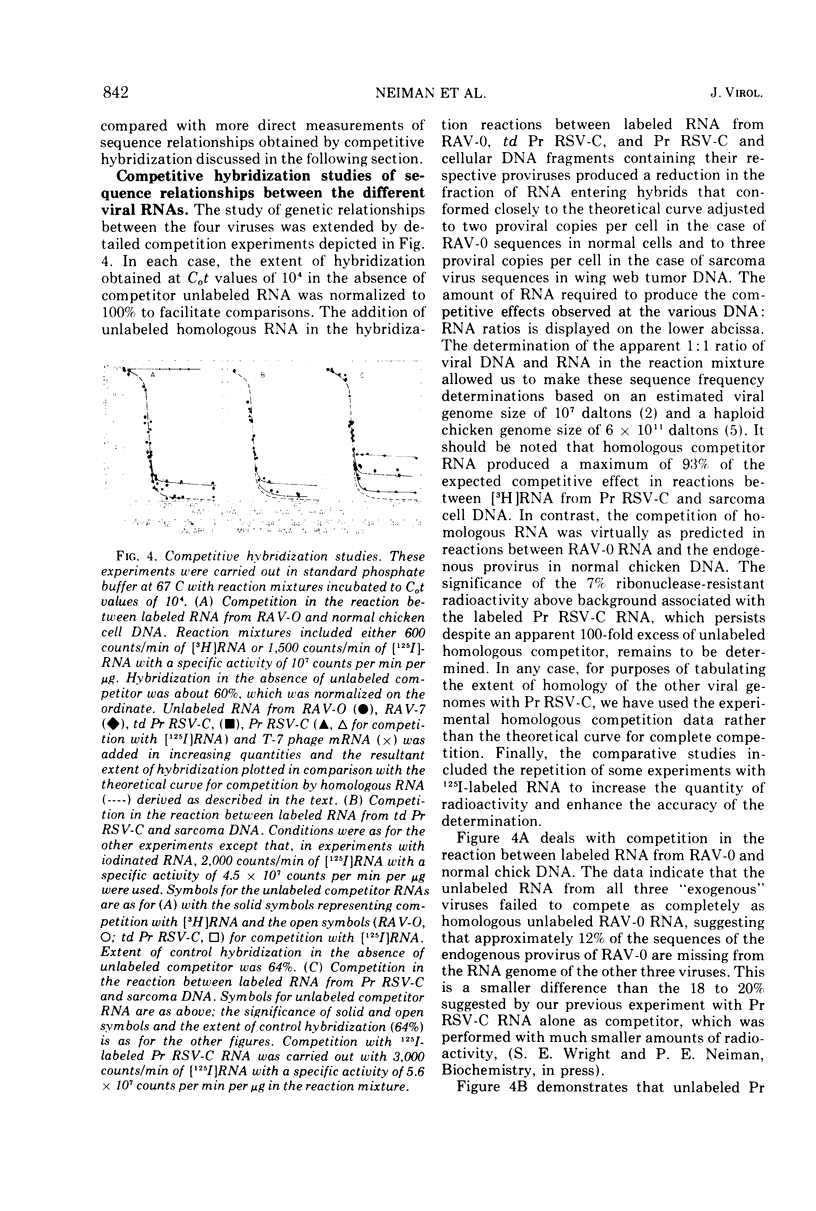
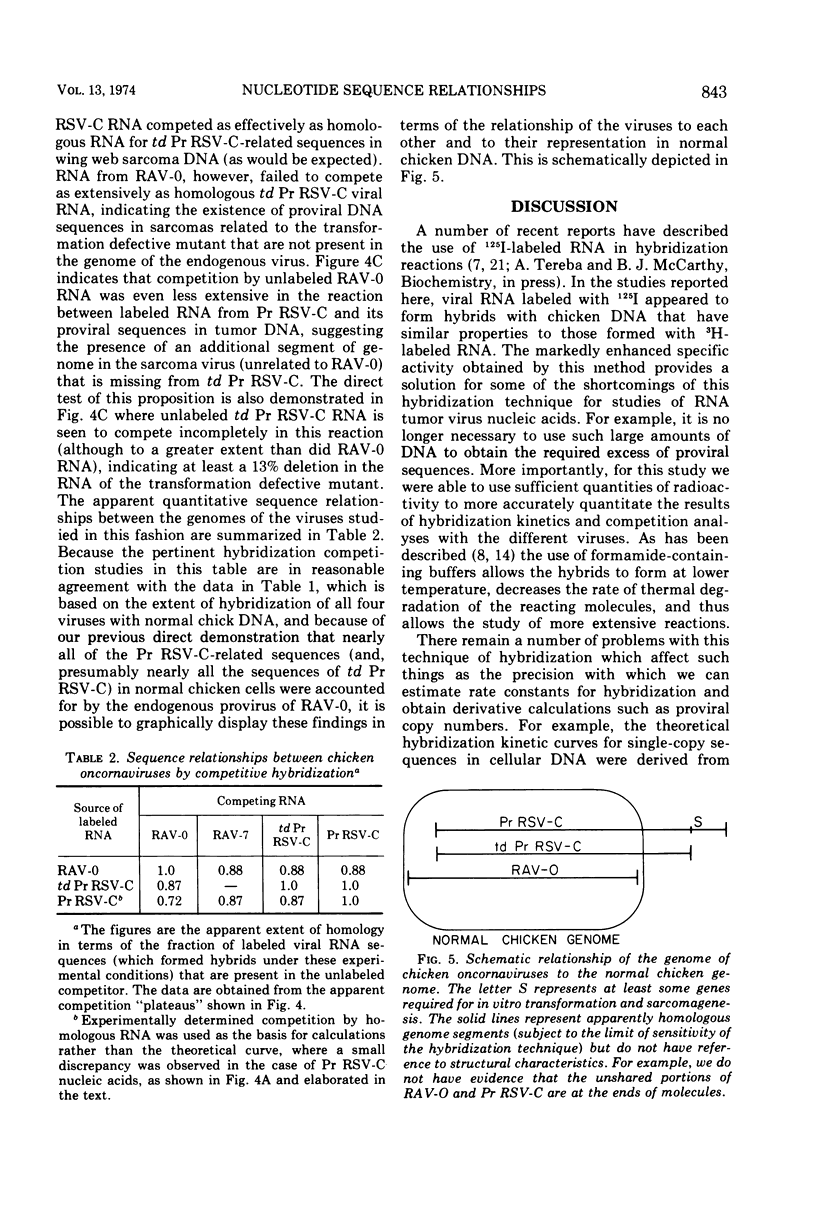



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BONAR R. A., BEARD J. W. Virus of avian myeloblastosis. XII. Chemical constitution. J Natl Cancer Inst. 1959 Jul;23(1):183–197. [PubMed] [Google Scholar]
- Biggs P. M., Milne B. S., Graf T., Bauer H. Oncogenicity of non-transforming mutants of avian sarcoma viruses. J Gen Virol. 1973 Mar;18(3):399–403. doi: 10.1099/0022-1317-18-3-399. [DOI] [PubMed] [Google Scholar]
- Britten R. J., Kohne D. E. Repeated sequences in DNA. Hundreds of thousands of copies of DNA sequences have been incorporated into the genomes of higher organisms. Science. 1968 Aug 9;161(3841):529–540. doi: 10.1126/science.161.3841.529. [DOI] [PubMed] [Google Scholar]
- Commerford S. L. Iodination of nucleic acids in vitro. Biochemistry. 1971 May 25;10(11):1993–2000. doi: 10.1021/bi00787a005. [DOI] [PubMed] [Google Scholar]
- Gelderman A. H., Rake A. V., Britten R. J. Transcription of nonrepeated DNA in neonatal and fetal mice. Proc Natl Acad Sci U S A. 1971 Jan;68(1):172–176. doi: 10.1073/pnas.68.1.172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Getz M. J., Altenburg L. C., Saunders G. F. The use of RNA labeled in vitro with iodine-125 in molecular hybridization experiments. Biochim Biophys Acta. 1972 Dec 22;287(3):485–494. doi: 10.1016/0005-2787(72)90293-6. [DOI] [PubMed] [Google Scholar]
- Grouse L., Chilton M. D., McCarthy B. J. Hybridization of ribonucleic acid with unique sequences of mouse deoxyribonucleic acid. Biochemistry. 1972 Feb 29;11(5):798–805. doi: 10.1021/bi00755a019. [DOI] [PubMed] [Google Scholar]
- Hill M., Hillova J. Virus recovery in chicken cells tested with Rous sarcoma cell DNA. Nat New Biol. 1972 May 10;237(71):35–39. doi: 10.1038/newbio237035a0. [DOI] [PubMed] [Google Scholar]
- Hutton J. R., Wetmur J. G. Renaturation of bacteriophage phiX174 DNA-RNA hybrid: RNA length effect and nucleation rate constant. J Mol Biol. 1973 Jul 15;77(4):495–500. doi: 10.1016/0022-2836(73)90218-0. [DOI] [PubMed] [Google Scholar]
- Lai M. M., Duesberg P. H., Horst J., Vogt P. K. Avian tumor virus RNA: a comparison of three sarcoma viruses and their transformation-defective derivatives by oligonucleotide fingerprinting and DNA-RNA hybridization. Proc Natl Acad Sci U S A. 1973 Aug;70(8):2266–2270. doi: 10.1073/pnas.70.8.2266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laird C. D., McConaughy B. L., McCarthy B. J. Rate of fixation of nucleotide substitutions in evolution. Nature. 1969 Oct 11;224(5215):149–154. doi: 10.1038/224149a0. [DOI] [PubMed] [Google Scholar]
- McConaughy B. L., Laird C. D., McCarthy B. J. Nucleic acid reassociation in formamide. Biochemistry. 1969 Aug;8(8):3289–3295. doi: 10.1021/bi00836a024. [DOI] [PubMed] [Google Scholar]
- Melli M., Whitfield C., Rao K. V., Richardson M., Bishop J. O. DNA-RNA hybridization in vast DNA excess. Nat New Biol. 1971 May 5;231(18):8–12. [PubMed] [Google Scholar]
- Neiman P. E. Measurement of endogenous leukosis virus nucleotide sequences in the DNA of normal avian embryos by RNA-DNA hybridization. Virology. 1973 May;53(1):196–203. doi: 10.1016/0042-6822(73)90478-9. [DOI] [PubMed] [Google Scholar]
- Neiman P. E. Rous sarcoma virus nucleotide sequences in cellular DNA: measurement by RNA-DNA hybridization. Science. 1972 Nov 17;178(4062):750–753. doi: 10.1126/science.178.4062.750. [DOI] [PubMed] [Google Scholar]
- Prensky W., Steffensen D. M., Hughes W. L. The use of iodinated RNA for gene localization. Proc Natl Acad Sci U S A. 1973 Jun;70(6):1860–1864. doi: 10.1073/pnas.70.6.1860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson W. S., Pitkanen A., Rubin H. The nucleic acid of the Bryan strain of Rous sarcoma virus: purification of the virus and isolation of the nucleic acid. Proc Natl Acad Sci U S A. 1965 Jul;54(1):137–144. doi: 10.1073/pnas.54.1.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubin H. A VIRUS IN CHICK EMBRYOS WHICH INDUCES RESISTANCE IN VITRO TO INFECTION WITH ROUS SARCOMA VIRUS. Proc Natl Acad Sci U S A. 1960 Aug;46(8):1105–1119. doi: 10.1073/pnas.46.8.1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Straus N. A., Bonner T. I. Temperature dependence of RNA-DNA hybridization kinetics. Biochim Biophys Acta. 1972 Aug 16;277(1):87–95. doi: 10.1016/0005-2787(72)90355-3. [DOI] [PubMed] [Google Scholar]
- Todaro G. J., Huebner R. J. N.A.S. symposium: new evidence as the basis for increased efforts in cancer research. Proc Natl Acad Sci U S A. 1972 Apr;69(4):1009–1015. doi: 10.1073/pnas.69.4.1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogt P. K., Friis R. R. An avian leukosis virus related to RSV(O): properties and evidence for helper activity. Virology. 1971 Jan;43(1):223–234. doi: 10.1016/0042-6822(71)90240-6. [DOI] [PubMed] [Google Scholar]
- Vogt P. K. Spontaneous segregation of nontransforming viruses from cloned sarcoma viruses. Virology. 1971 Dec;46(3):939–946. doi: 10.1016/0042-6822(71)90092-4. [DOI] [PubMed] [Google Scholar]
- Weiss R. A., Friis R. R., Katz E., Vogt P. K. Induction of avian tumor viruses in normal cells by physical and chemical carcinogens. Virology. 1971 Dec;46(3):920–938. doi: 10.1016/0042-6822(71)90091-2. [DOI] [PubMed] [Google Scholar]


