Abstract
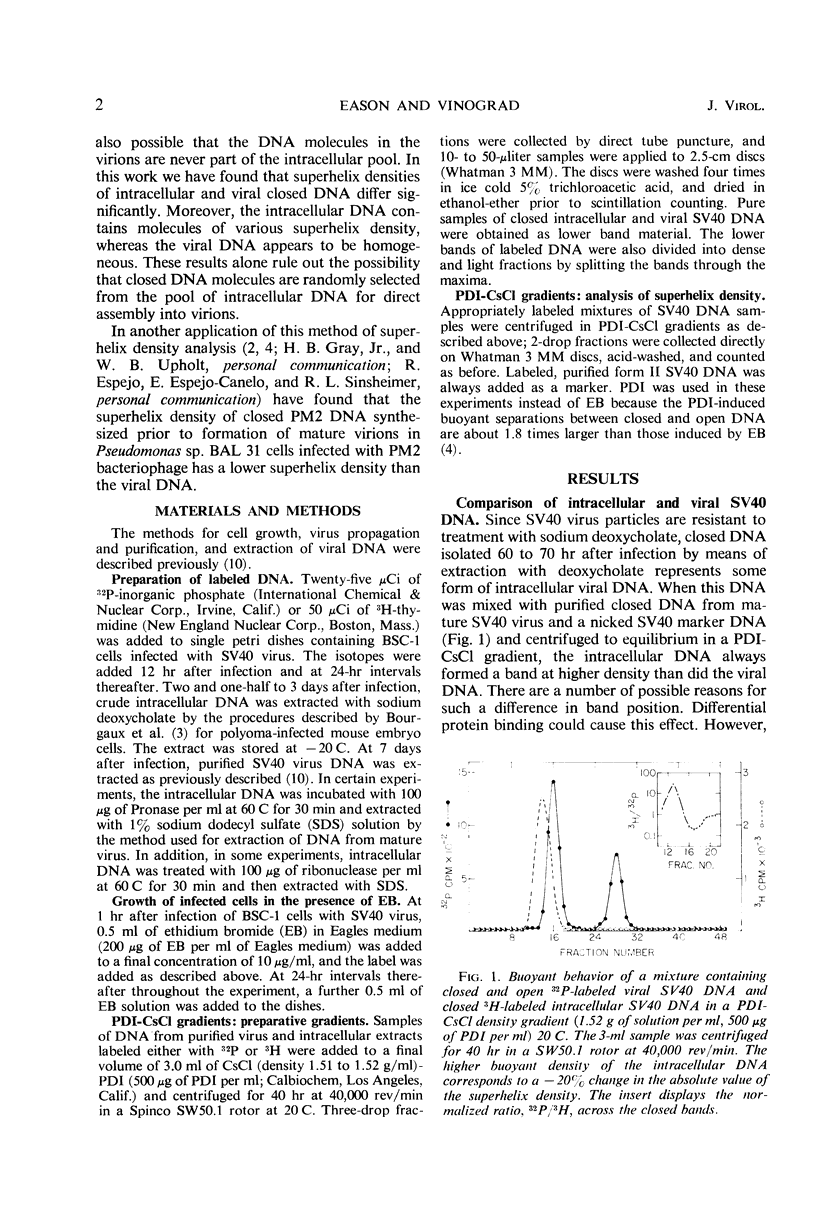
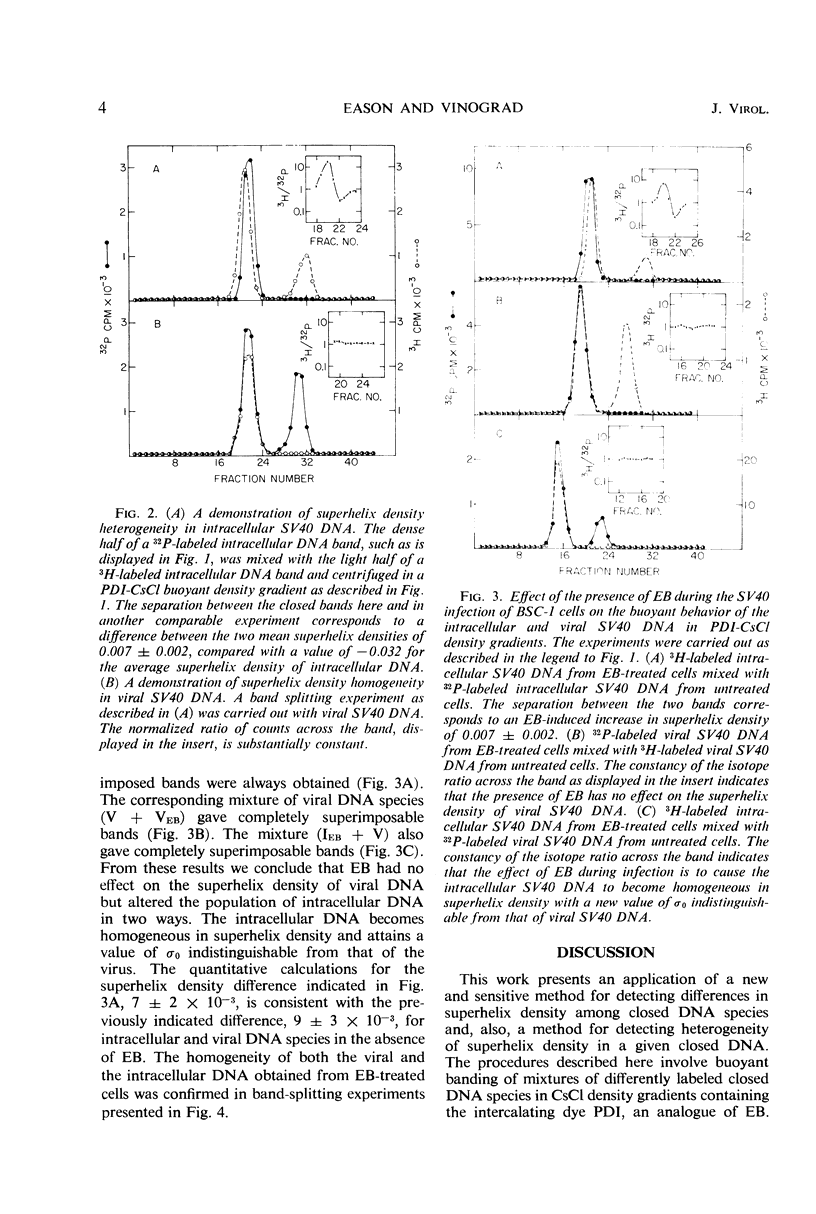
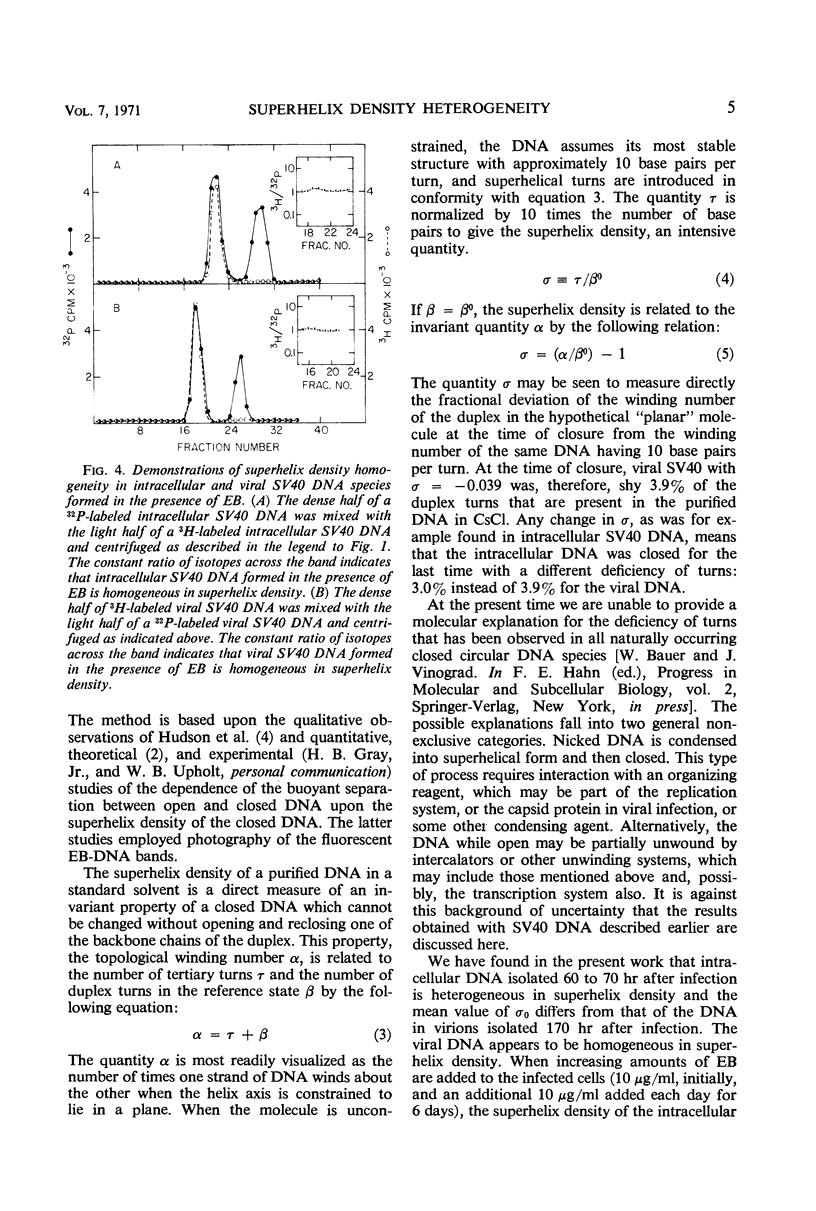
Covalently closed intracellular and viral simian virus 40 (SV40) deoxyribonucleic acid (DNA) were separately isolated from infected African green monkey cells (BSC-1) grown in culture. The two DNA species form overlapping bands centered at different positions in a propidium di-iodide-cesium chloride (PDI-CsCl) buoyant density gradient capable of separating closed DNA species with different superhelix densities. When the dense side of a 32P-labeled intracellular DNA band was mixed with the light side of a 3H-labeled intracellular DNA band and again centrifuged in a PDI-CsCl density gradient, two overlapping bands formed with modes displaced from each other. Similar band-splitting experiments performed with viral DNA always gave superimposable bands. The foregoing experiments demonstrate that the intracellular DNA is heterogeneous in superhelix density, whereas, by the same criteria, the viral DNA is homogeneous. The mean superhelix density of the intracellular closed DNA is approximately three-fourths as large as the superhelix density of the viral DNA. These results rule out the possibility that closed SV40 DNA is drawn randomly from the intracellular pool and assembled without a further nicking-closing step into virions. When the cells were grown and infected in the presence of ethidium bromide (EB), the intracellular closed DNA was found to be homogeneous in superhelix density and to have the same superhelix density as the viral DNA which, in turn, was unaffected by the presence of the drug. The foregoing results were explained by postulating that the intracellular DNA is formed with a homogeneous superhelix density and becomes heterogeneous in the absence of EB as a result of a nicking-closing cycle that occurs in a spacially or temporally heterogeneous environment. The drug EB would inhibit this action by inhibiting the nicking enzyme(s).
Full text
PDF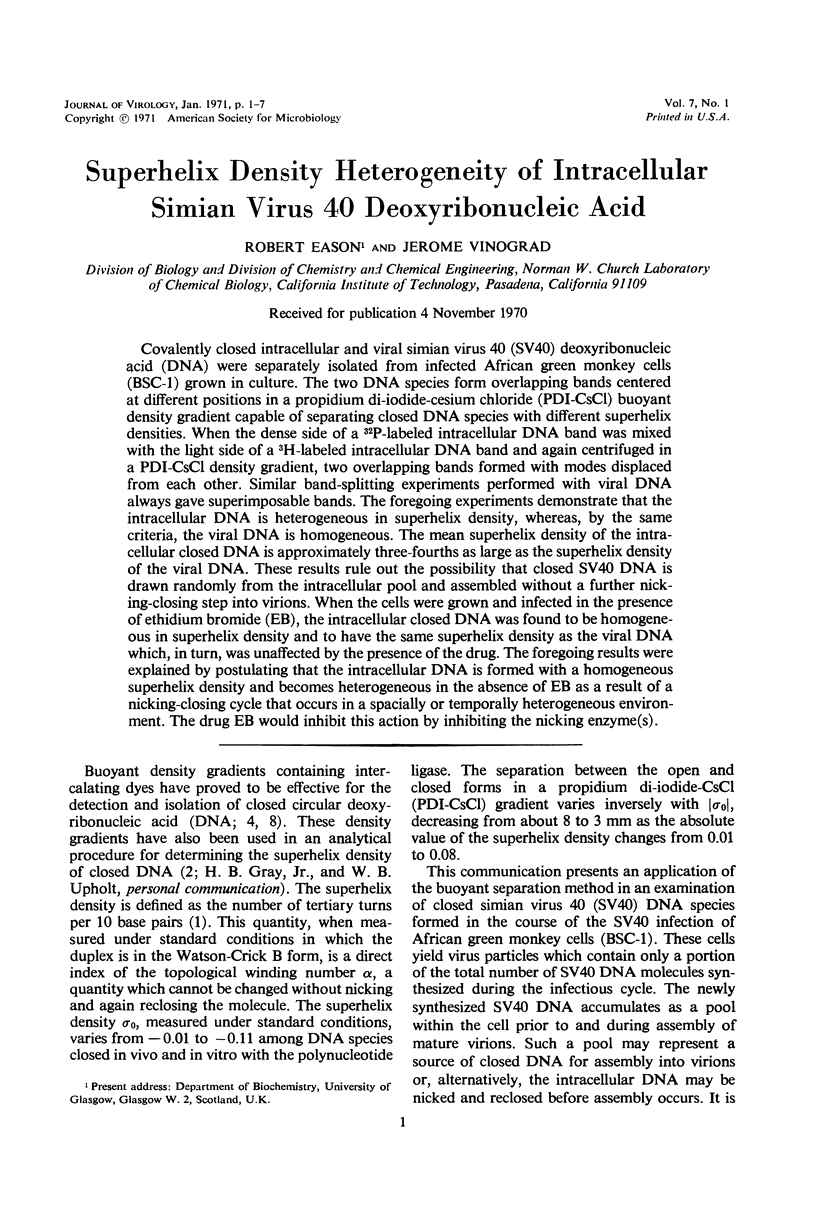



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bauer W., Vinograd J. The interaction of closed circular DNA with intercalative dyes. I. The superhelix density of SV40 DNA in the presence and absence of dye. J Mol Biol. 1968 Apr 14;33(1):141–171. doi: 10.1016/0022-2836(68)90286-6. [DOI] [PubMed] [Google Scholar]
- Bourgaux P., Bourgaux-Ramoisy D., Dulbecco R. The replication of the ring-shaped DNA of polyoma virus. I. Identification of the replicative intermediate. Proc Natl Acad Sci U S A. 1969 Oct;64(2):701–708. doi: 10.1073/pnas.64.2.701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hudson B., Upholt W. B., Devinny J., Vinograd J. The use of an ethidium analogue in the dye-buoyant density procedure for the isolation of closed circular DNA: the variation of the superhelix density of mitochondrial DNA. Proc Natl Acad Sci U S A. 1969 Mar;62(3):813–820. doi: 10.1073/pnas.62.3.813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine A. J., Kang H. S., Billheimer F. E. DNA replication in SV40 infected cells. I. Analysis of replicating SV40 DNA. J Mol Biol. 1970 Jun 14;50(2):549–568. doi: 10.1016/0022-2836(70)90211-1. [DOI] [PubMed] [Google Scholar]
- Levine A. J., Teresky A. K. Deoxyribonucleic acid replication in simian virus 40-infected cells. II. Detection and characterization of simian virus 40 pseudovirions. J Virol. 1970 Apr;5(4):451–457. doi: 10.1128/jvi.5.4.451-457.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer R. R., Simpson M. V. DNA biosynthesis in mitochondria. Differential inhibition of mitochondrial and nuclear DNA polymerases by the mutagenic dyes ethidium bromide and acriflavin. Biochem Biophys Res Commun. 1969 Jan 27;34(2):238–244. doi: 10.1016/0006-291x(69)90637-8. [DOI] [PubMed] [Google Scholar]
- Radloff R., Bauer W., Vinograd J. A dye-buoyant-density method for the detection and isolation of closed circular duplex DNA: the closed circular DNA in HeLa cells. Proc Natl Acad Sci U S A. 1967 May;57(5):1514–1521. doi: 10.1073/pnas.57.5.1514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritzi E., Levine A. J. Deoxyribonucleic acid replication in simian virus 40-infected cells. 3. Comparison of simian virus 40 lytic infection in three different monkey kidney cell lines. J Virol. 1970 Jun;5(6):686–692. doi: 10.1128/jvi.5.6.686-692.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]


