Abstract
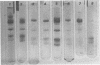
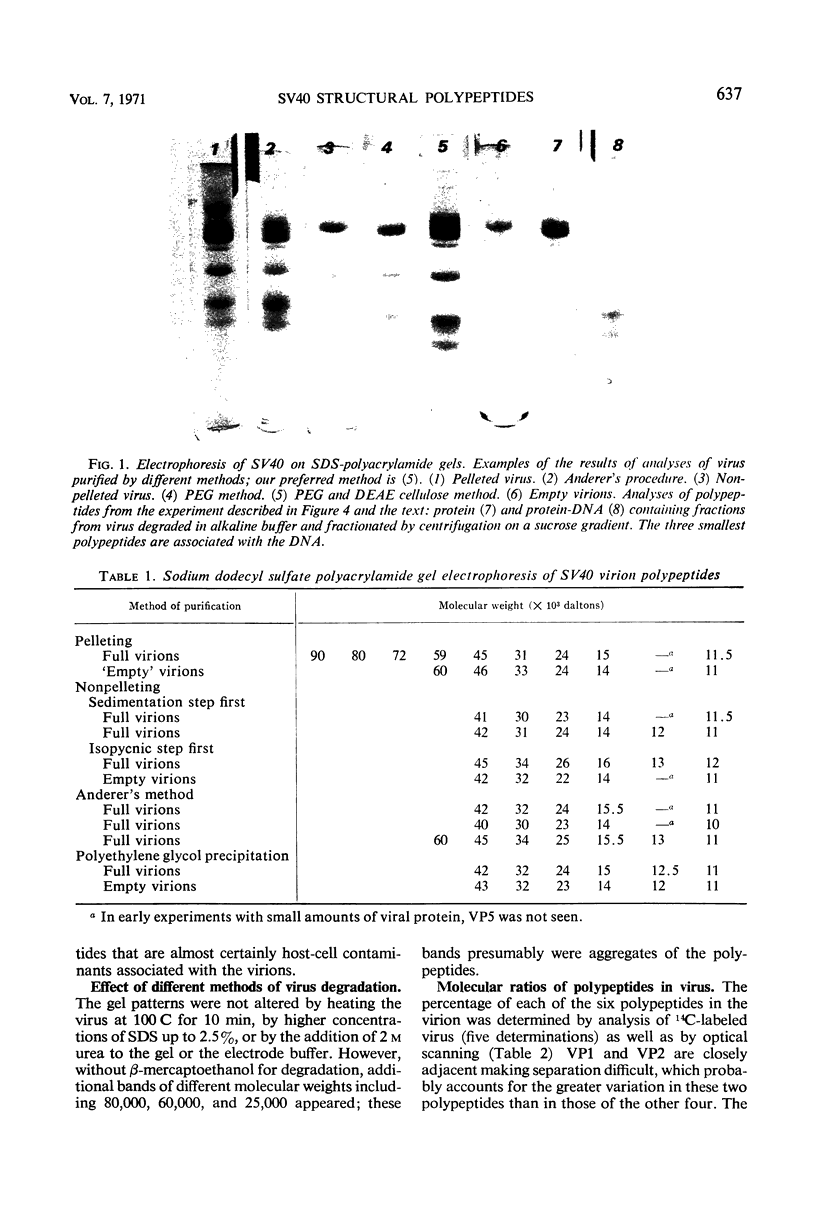
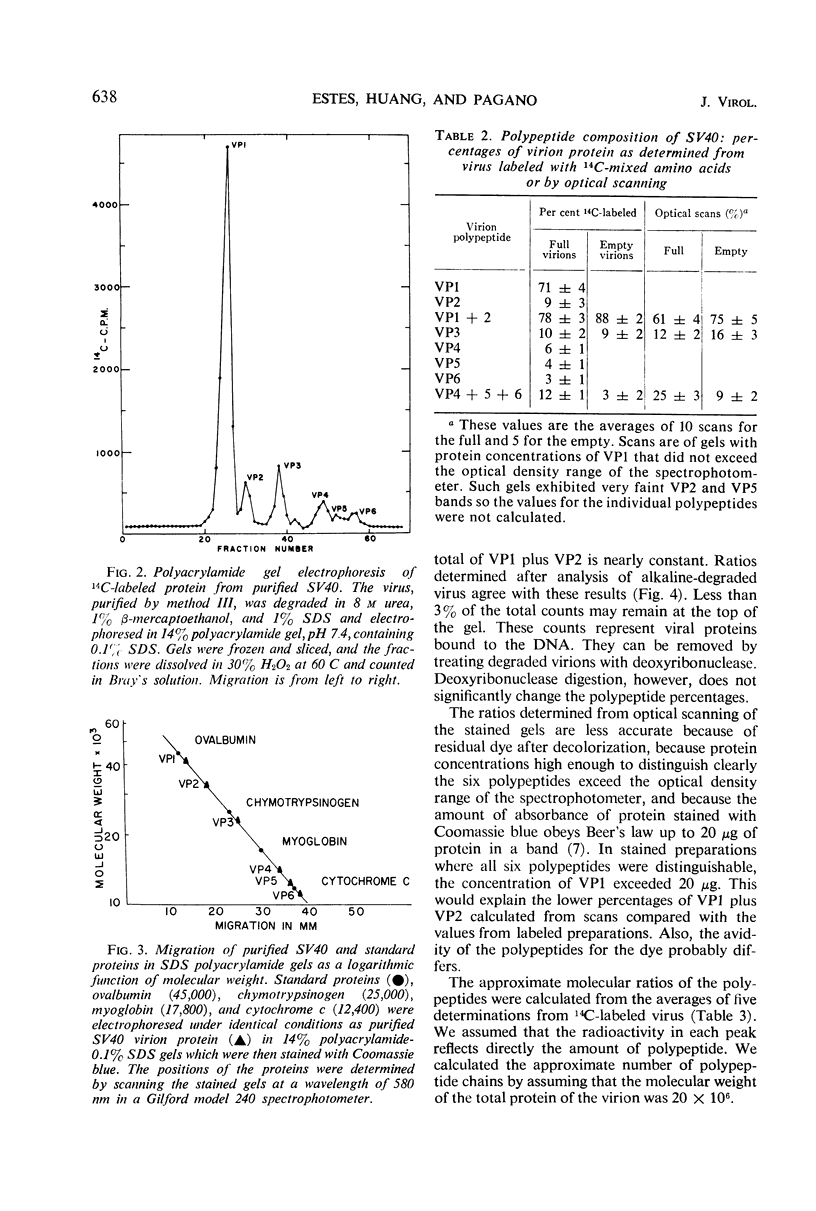
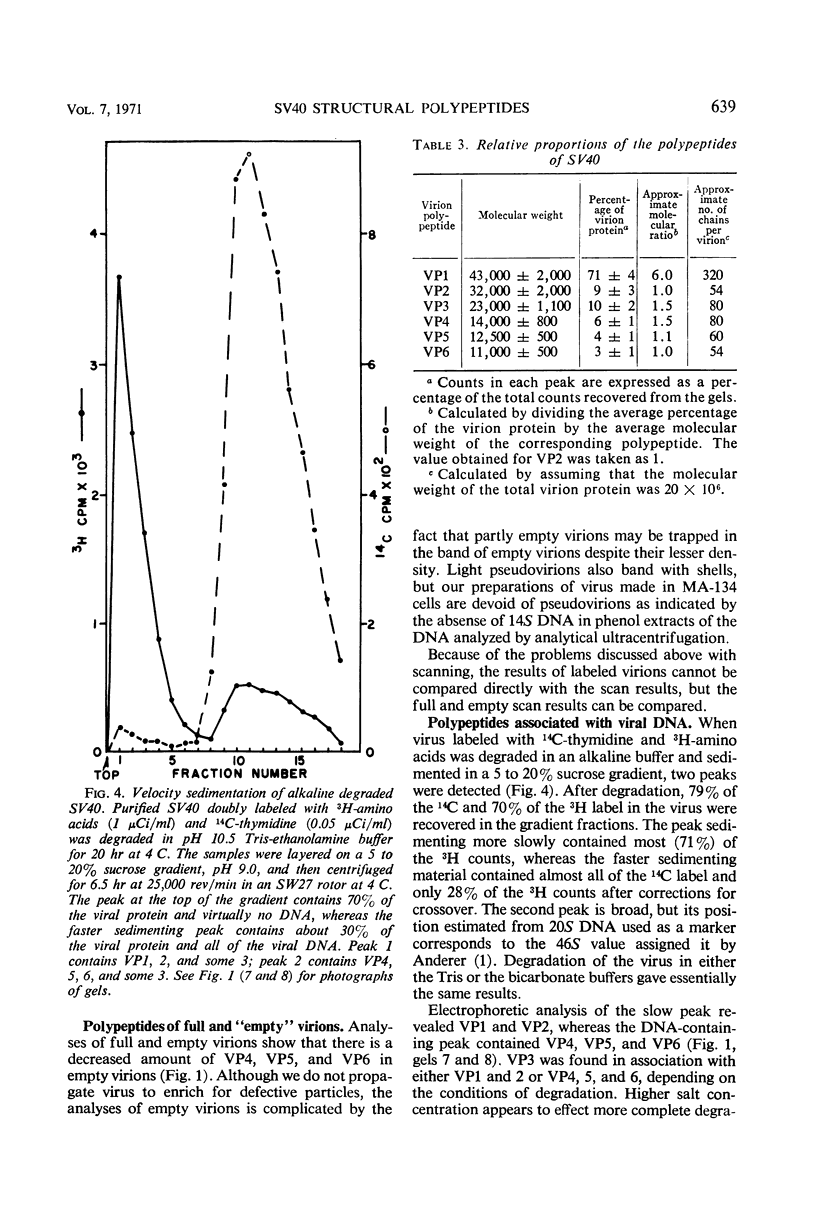
To determine the number and molecular weights of the structural polypeptides of simian virus 40, we have analyzed purified virus by electrophoresis on 14% polyacrylamide gels containing sodium dodecyl sulfate. Full virus purified by several different methods showed six distinct bands with molecular weights of approximately 43,000 (VP1, containing 70% of virion protein), 32,000 (VP2, 9%), 23,000 (VP3, 10%), 14,000 (VP4, 6%), 12,500 (VP5, 4%), and 11,000 (VP6, 3%) both by analysis of radioactively labeled virions and by visualization of the polypeptide bands after staining. “Empty” virions contain decreased amounts of VP4, 5, and 6. The approximate molecular ratios of the polypeptides were 6.0, 1.0, 1.5, 1.5, 1.1, and 1.0. When virus degraded in an alkaline buffer was analyzed by velocity centrifugation in sucrose gradients, the two larger polypeptides (VP1 and VP2) remained at the top of the gradient, whereas the three smallest polypeptides (VP4, 5, and 6) sedimented as a complex with the viral deoxyribonucleic acid. VP3 was found in association with either VP1 and 2 or VP4, 5, and 6, depending on the conditions of degradation. Presumably, VP1 and VP2, comprising about 80% of the protein, form the capsid of the virus. VP4, 5, and 6 may form a nucleoprotein in the virion, and VP3 may serve as an intermediate structural component.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderer F. A., Koch M. A., Schlumberger H. D. Structure of simian virus 40. 3. Alkaline degradation of the virus particle. Virology. 1968 Mar;34(3):452–458. doi: 10.1016/0042-6822(68)90065-2. [DOI] [PubMed] [Google Scholar]
- Anderer F. A., Schlumberger H. D., Koch M. A., Frank H., Eggers H. J. Structure of simian virus 40. II. Symmetry and components of the virus particle. Virology. 1967 Jul;32(3):511–523. doi: 10.1016/0042-6822(67)90303-0. [DOI] [PubMed] [Google Scholar]
- BLACK P. H., CRAWFORD E. M., CRAWFORD L. V. THE PURIFICATION OF SIMIAN VIRUS 40. Virology. 1964 Nov;24:381–387. doi: 10.1016/0042-6822(64)90175-8. [DOI] [PubMed] [Google Scholar]
- CRAWFORD L. V., BLACK P. H. THE NUCLEIC ACID OF SIMIAN VIRUS 40. Virology. 1964 Nov;24:388–392. doi: 10.1016/0042-6822(64)90176-x. [DOI] [PubMed] [Google Scholar]
- Dunker A. K., Rueckert R. R. Observations on molecular weight determinations on polyacrylamide gel. J Biol Chem. 1969 Sep 25;244(18):5074–5080. [PubMed] [Google Scholar]
- Fambrough D. M., Bonner J. Sequence homology and role of cysteine in plant and animal arginine-rich histones. J Biol Chem. 1968 Sep 10;243(17):4434–4439. [PubMed] [Google Scholar]
- Friedmann T., Haas M. Rapid concentration and purification of polyoma virus and SV40 with polyethylene glycol. Virology. 1970 Sep;42(1):248–250. doi: 10.1016/0042-6822(70)90263-1. [DOI] [PubMed] [Google Scholar]
- Girard M., Marty L., Suarez F. Capsid proteins of Simian virus 40. Biochem Biophys Res Commun. 1970 Jul 13;40(1):97–102. doi: 10.1016/0006-291x(70)91051-x. [DOI] [PubMed] [Google Scholar]
- Iwai K., Ishikawa K., Hayashi H. Amino-acid sequence of slightly lysine-rich histone. Nature. 1970 Jun 13;226(5250):1056–1058. doi: 10.1038/2261056b0. [DOI] [PubMed] [Google Scholar]
- Kass S. J. Chemical studies on polyoma and Shope papilloma viruses. J Virol. 1970 Mar;5(3):381–387. doi: 10.1128/jvi.5.3.381-387.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch M. A., Eggers H. J., Anderer F. A., Schlumberger H. D., Frank H. Structure of simian virus 40. I. Purification and physical characterization of the virus particle. Virology. 1967 Jul;32(3):503–510. doi: 10.1016/0042-6822(67)90302-9. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Levine A. J., Teresky A. K. Deoxyribonucleic acid replication in simian virus 40-infected cells. II. Detection and characterization of simian virus 40 pseudovirions. J Virol. 1970 Apr;5(4):451–457. doi: 10.1128/jvi.5.4.451-457.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michel M. R., Hirt B., Weil R. Mouse cellular DNA enclosed in polyoma viral capsids (pseudovirions). Proc Natl Acad Sci U S A. 1967 Oct;58(4):1381–1388. doi: 10.1073/pnas.58.4.1381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shapiro A. L., Viñuela E., Maizel J. V., Jr Molecular weight estimation of polypeptide chains by electrophoresis in SDS-polyacrylamide gels. Biochem Biophys Res Commun. 1967 Sep 7;28(5):815–820. doi: 10.1016/0006-291x(67)90391-9. [DOI] [PubMed] [Google Scholar]
- Teller D. C., Kinkade J. M., Jr, Cole R. D. The molecular weight of lysine-rich histone. Biochem Biophys Res Commun. 1965 Sep 22;20(6):739–744. doi: 10.1016/0006-291x(65)90079-3. [DOI] [PubMed] [Google Scholar]
- Winocour E. Some aspects of the interaction between polyoma virus and cell DNA. Adv Virus Res. 1969;14:153–200. doi: 10.1016/s0065-3527(08)60559-x. [DOI] [PubMed] [Google Scholar]