Abstract
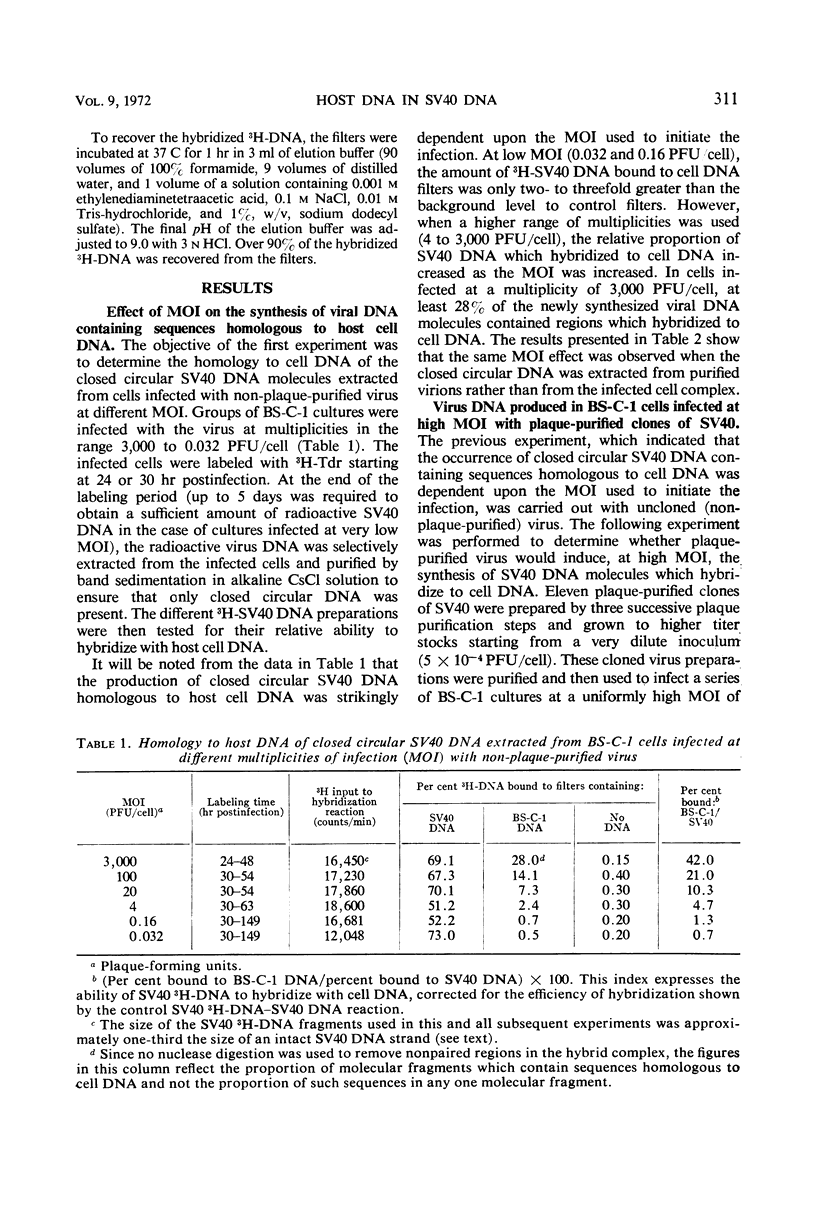
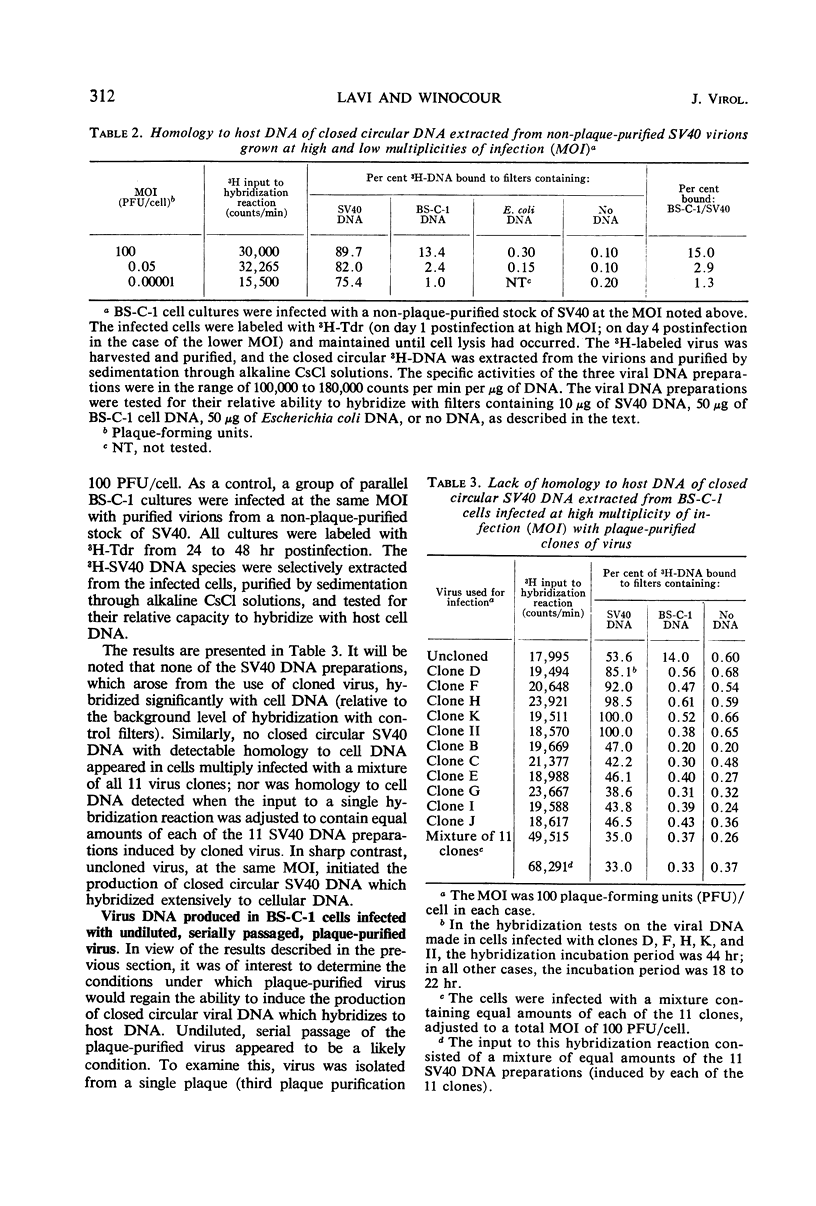
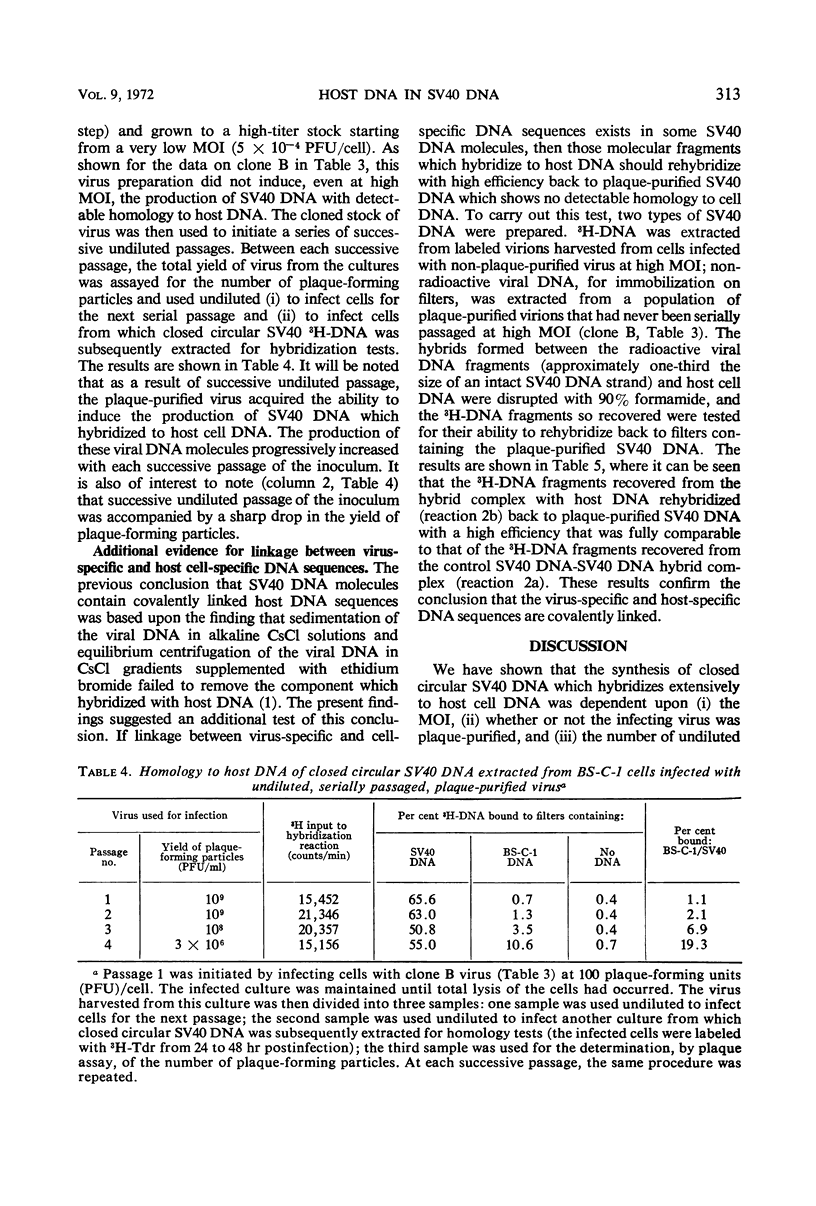
The synthesis of closed circular simian virus 40 (SV40) deoxyribonucleic acid (DNA) containing sequences homologous to host cell DNA depends upon the conditions under which the cells are infected. When BS-C-1 monkey cells were infected with non-plaque-purified virus at low multiplicity of infection [MOI, 0.032 plaque-forming units (PFU)/cell], little, if any, of the SV40 DNA extracted from the infected cells hybridized to host DNA; but when increasingly higher multiplicities were used (in the range 0.16 to 3,000 PFU/cell), an increasingly greater amount of the extracted SV40 DNA hybridized to host DNA. The same effect was observed when the closed circular SV40 DNA was extracted from purified virions (grown at low and high MOI) rather than from the infected cell complex. When the cells were infected at high MOI with plaque-purified virus (11 viral clones were tested), none of the SV40 DNA extracted from the cells hybridized detectably with host cell DNA. However, plaque-purified virus that was serially passaged, undiluted, induced the synthesis of virus DNA which again showed extensive homology to host DNA. It is suggested that, under certain circumstances, recombination occurs between viral and host DNA during lytic infection which results in the incorporation of host DNA sequences into closed circular SV40 DNA.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aloni Y., Winocour E., Sachs L., Torten J. Hybridization between SV40 DNA and cellular DNA's. J Mol Biol. 1969 Sep 14;44(2):333–345. doi: 10.1016/0022-2836(69)90179-x. [DOI] [PubMed] [Google Scholar]
- BLACK P. H., CRAWFORD E. M., CRAWFORD L. V. THE PURIFICATION OF SIMIAN VIRUS 40. Virology. 1964 Nov;24:381–387. doi: 10.1016/0042-6822(64)90175-8. [DOI] [PubMed] [Google Scholar]
- Britten R. J., Kohne D. E. Repeated sequences in DNA. Hundreds of thousands of copies of DNA sequences have been incorporated into the genomes of higher organisms. Science. 1968 Aug 9;161(3841):529–540. doi: 10.1126/science.161.3841.529. [DOI] [PubMed] [Google Scholar]
- Denhardt D. T. A membrane-filter technique for the detection of complementary DNA. Biochem Biophys Res Commun. 1966 Jun 13;23(5):641–646. doi: 10.1016/0006-291x(66)90447-5. [DOI] [PubMed] [Google Scholar]
- GERBER P. An infectious deoxyribonucleic acid derived from vacuolating virus (SV40). Virology. 1962 Jan;16:96–97. doi: 10.1016/0042-6822(62)90209-x. [DOI] [PubMed] [Google Scholar]
- Gelb L. D., Kohne D. E., Martin M. A. Quantitation of Simian virus 40 sequences in African green monkey, mouse and virus-transformed cell genomes. J Mol Biol. 1971 Apr 14;57(1):129–145. doi: 10.1016/0022-2836(71)90123-9. [DOI] [PubMed] [Google Scholar]
- Gershon D., Sachs L., Winocour E. The induction of cellular DNA synthesis by simian virus 40 in contact-inhibited and in x-irradiated cells. Proc Natl Acad Sci U S A. 1966 Sep;56(3):918–925. doi: 10.1073/pnas.56.3.918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillespie D., Spiegelman S. A quantitative assay for DNA-RNA hybrids with DNA immobilized on a membrane. J Mol Biol. 1965 Jul;12(3):829–842. doi: 10.1016/s0022-2836(65)80331-x. [DOI] [PubMed] [Google Scholar]
- Grady L., Axelrod D., Trilling D. The SV40 pseudovirus: its potential for general transduction in animal cells. Proc Natl Acad Sci U S A. 1970 Dec;67(4):1886–1893. doi: 10.1073/pnas.67.4.1886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HOPPS H. E., BERNHEIM B. C., NISALAK A., TJIO J. H., SMADEL J. E. BIOLOGIC CHARACTERISTICS OF A CONTINUOUS KIDNEY CELL LINE DERIVED FROM THE AFRICAN GREEN MONKEY. J Immunol. 1963 Sep;91:416–424. [PubMed] [Google Scholar]
- Hirt B. Selective extraction of polyoma DNA from infected mouse cell cultures. J Mol Biol. 1967 Jun 14;26(2):365–369. doi: 10.1016/0022-2836(67)90307-5. [DOI] [PubMed] [Google Scholar]
- Levine A. J., Teresky A. K. Deoxyribonucleic acid replication in simian virus 40-infected cells. II. Detection and characterization of simian virus 40 pseudovirions. J Virol. 1970 Apr;5(4):451–457. doi: 10.1128/jvi.5.4.451-457.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McConaughy B. L., Laird C. D., McCarthy B. J. Nucleic acid reassociation in formamide. Biochemistry. 1969 Aug;8(8):3289–3295. doi: 10.1021/bi00836a024. [DOI] [PubMed] [Google Scholar]
- Melli M., Bishop J. O. Hybridization between rat liver DNA and complementary RNA. J Mol Biol. 1969 Feb 28;40(1):117–136. doi: 10.1016/0022-2836(69)90300-3. [DOI] [PubMed] [Google Scholar]
- Michel M. R., Hirt B., Weil R. Mouse cellular DNA enclosed in polyoma viral capsids (pseudovirions). Proc Natl Acad Sci U S A. 1967 Oct;58(4):1381–1388. doi: 10.1073/pnas.58.4.1381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radloff R., Bauer W., Vinograd J. A dye-buoyant-density method for the detection and isolation of closed circular duplex DNA: the closed circular DNA in HeLa cells. Proc Natl Acad Sci U S A. 1967 May;57(5):1514–1521. doi: 10.1073/pnas.57.5.1514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- STUDIER F. W. SEDIMENTATION STUDIES OF THE SIZE AND SHAPE OF DNA. J Mol Biol. 1965 Feb;11:373–390. doi: 10.1016/s0022-2836(65)80064-x. [DOI] [PubMed] [Google Scholar]
- Sambrook J., Westphal H., Srinivasan P. R., Dulbecco R. The integrated state of viral DNA in SV40-transformed cells. Proc Natl Acad Sci U S A. 1968 Aug;60(4):1288–1295. doi: 10.1073/pnas.60.4.1288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uchida S., Watanabe S., Kato M. Incomplete growth of simian virus 40 in African green monkey kidney culture induced by serial undiluted passages. Virology. 1966 Jan;28(1):135–141. doi: 10.1016/0042-6822(66)90314-x. [DOI] [PubMed] [Google Scholar]
- VINOGRAD J., BRUNER R., KENT R., WEIGLE J. Band-centrifugation of macromolecules and viruses in self-generating density gradients. Proc Natl Acad Sci U S A. 1963 Jun;49:902–910. doi: 10.1073/pnas.49.6.902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vinograd J., Lebowitz J., Radloff R., Watson R., Laipis P. The twisted circular form of polyoma viral DNA. Proc Natl Acad Sci U S A. 1965 May;53(5):1104–1111. doi: 10.1073/pnas.53.5.1104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WEIL R. A quantitative assay for a subviral infective agent related to polyoma virus. Virology. 1961 May;14:46–53. doi: 10.1016/0042-6822(61)90130-1. [DOI] [PubMed] [Google Scholar]
- Winocour E. Further studies on the incorporation of cell DNA into polyoma-related particles. Virology. 1968 Apr;34(4):571–582. doi: 10.1016/0042-6822(68)90078-0. [DOI] [PubMed] [Google Scholar]
- Winocour E. On the apparent homology between DNA from polyoma virus and normal mouse synthetic RNA. Virology. 1967 Jan;31(1):15–28. doi: 10.1016/0042-6822(67)90003-7. [DOI] [PubMed] [Google Scholar]
- Winocour E. The investigation of oncogeic viral genomes in transformed cells by nucleic acid hybridization. Adv Cancer Res. 1971;14:37–70. doi: 10.1016/s0065-230x(08)60518-7. [DOI] [PubMed] [Google Scholar]
- Yoshiike K. Studies on DNA from low-density particles of SV40. I. Heterogeneous defective virions produced by successive undiluted passages. Virology. 1968 Mar;34(3):391–401. doi: 10.1016/0042-6822(68)90059-7. [DOI] [PubMed] [Google Scholar]


