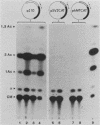
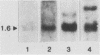
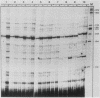
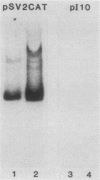
Abstract
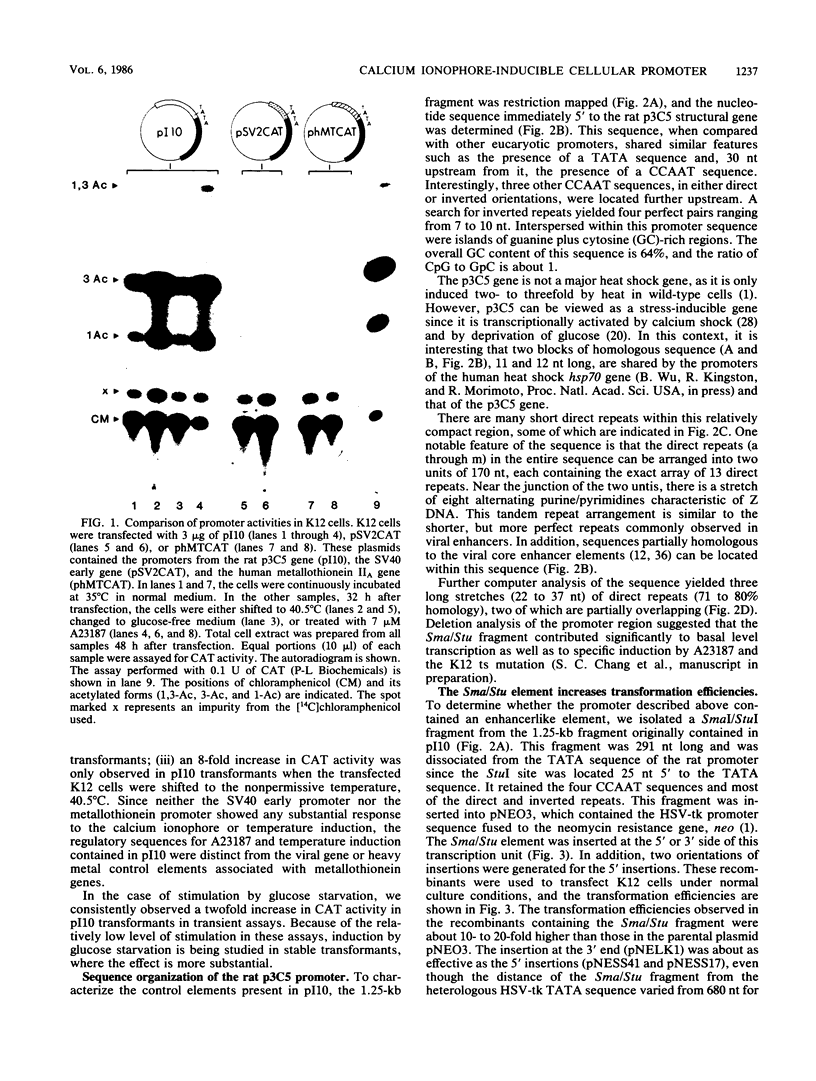
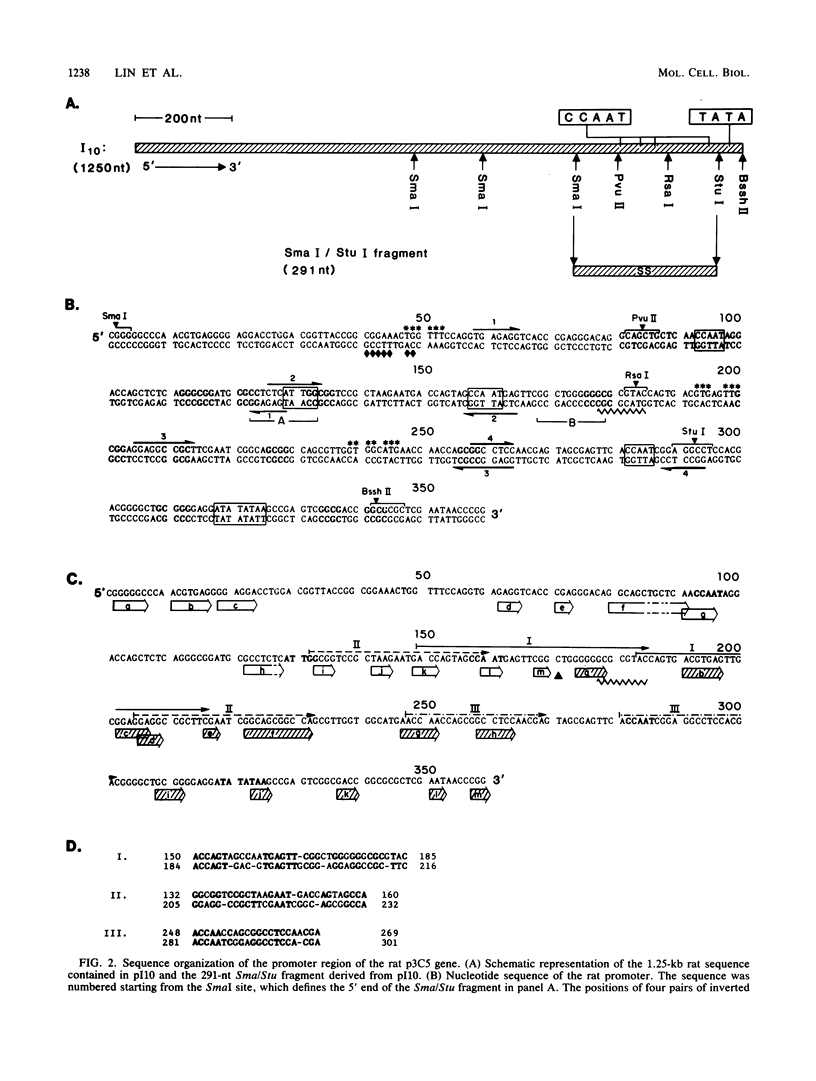
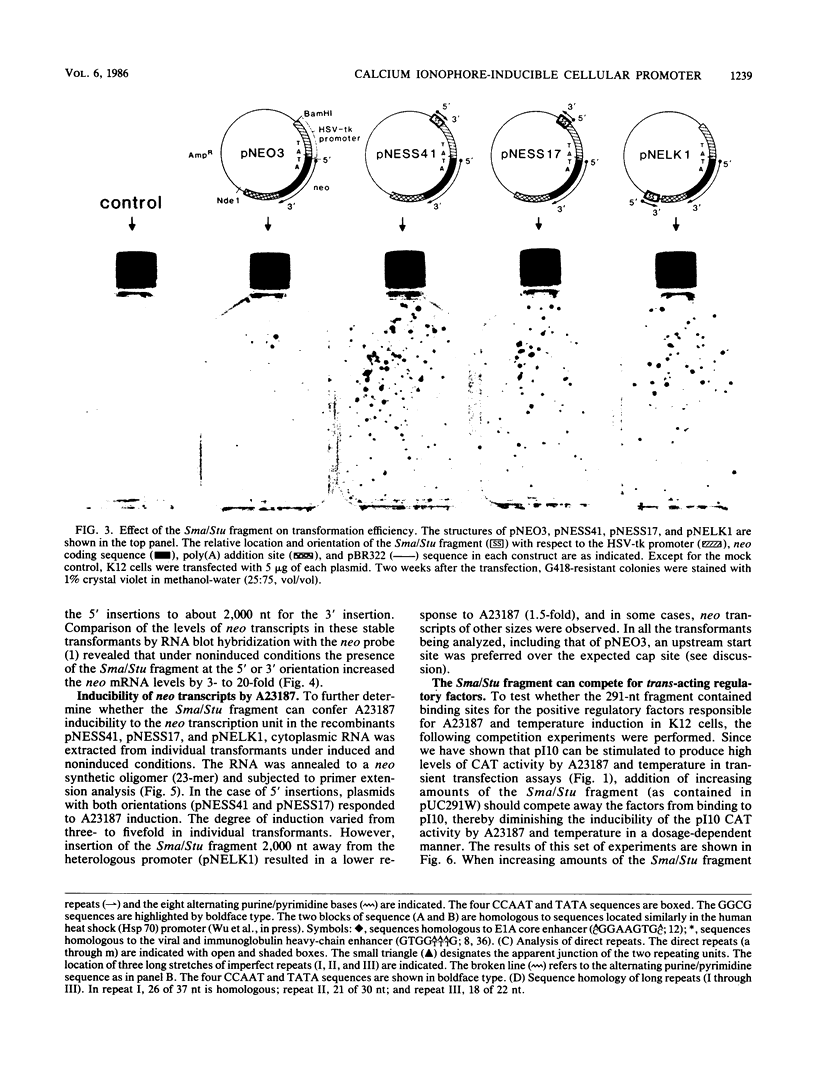
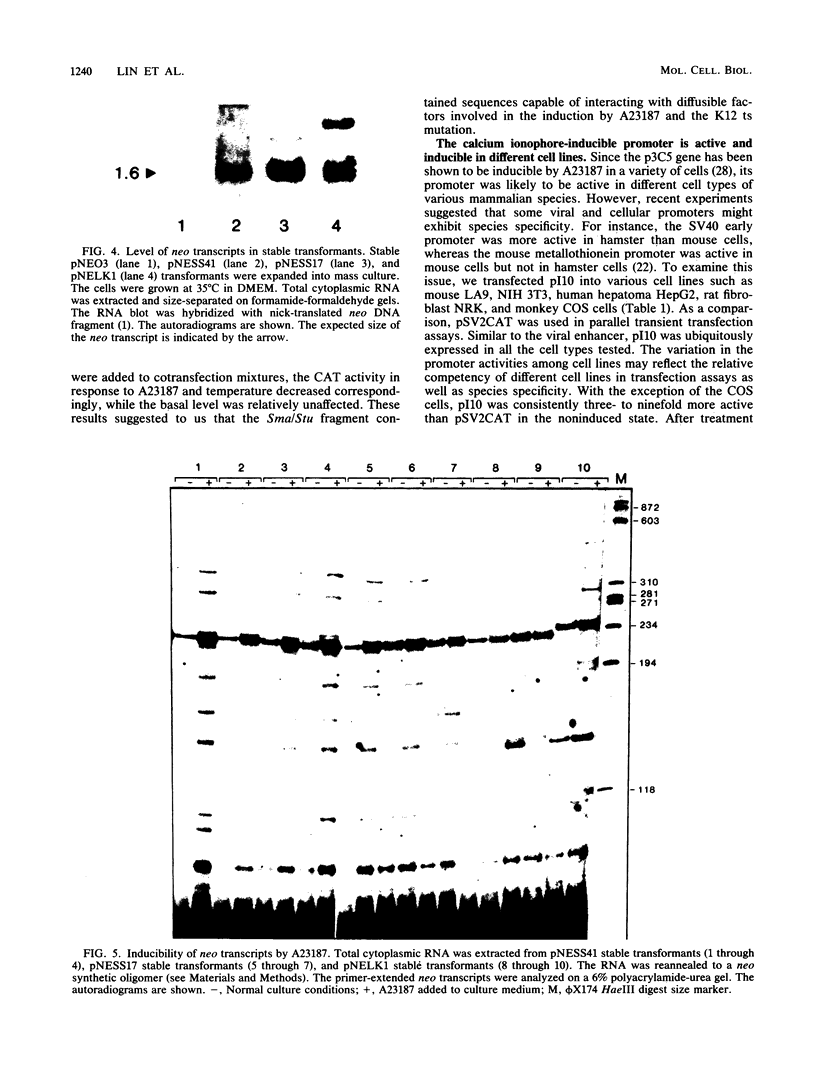
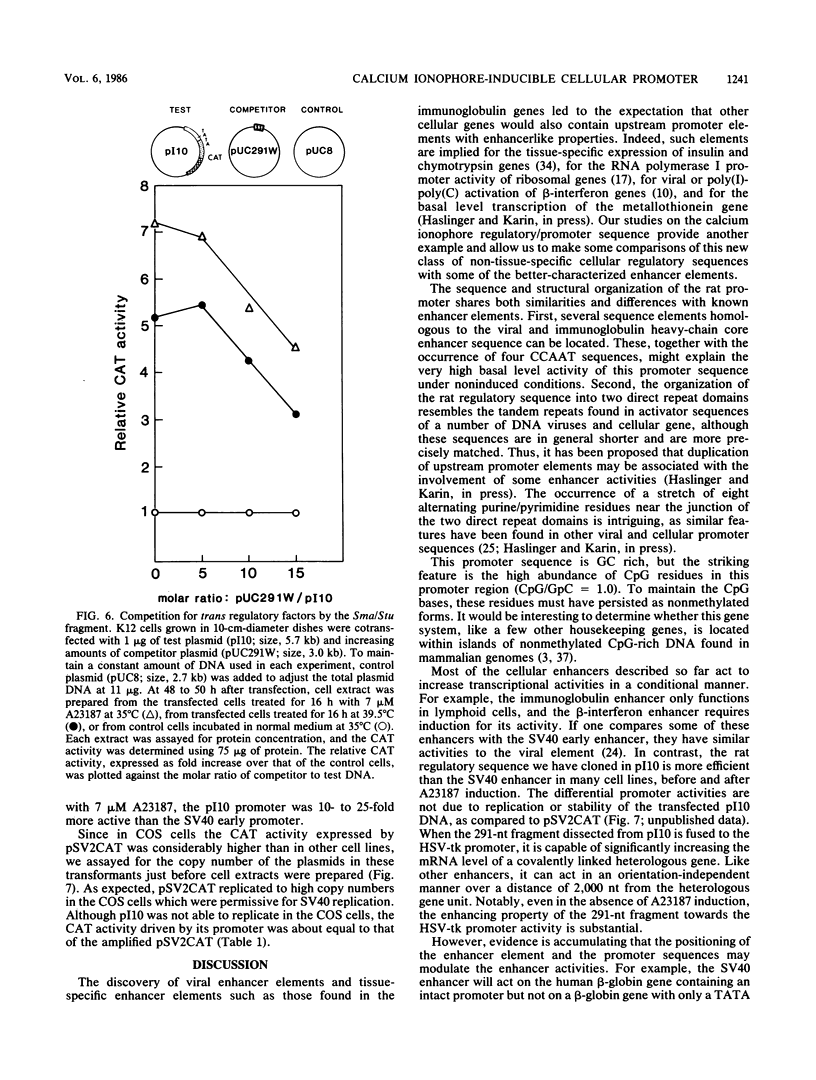
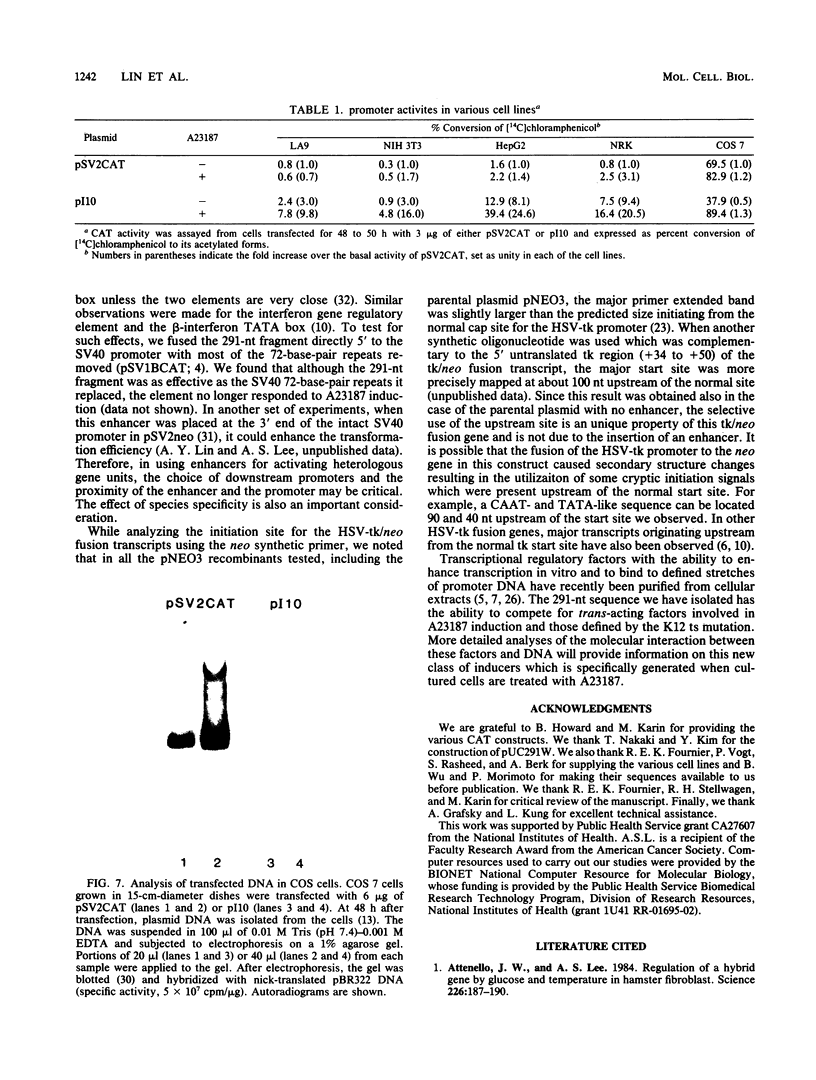
We examined the regulatory/promoter sequence of a calcium ionophore-inducible gene isolated from the rat genome. Whereas the promoter of this ubiquitously expressed gene is active under noninduced conditions, after induction by calcium ionophore A23187 this promoter is 10- to 25-fold more active than the simian virus 40 early promoter, as measured by chloramphenicol acetyltransferase activities. Within this regulatory/promoter region, we have identified a DNA fragment with enhancer-like properties immediately 5' to the TATA sequence. This 291-nucleotide fragment acts in cis to enhance expression of the neomycin phosphotransferase (neo) gene driven by the herpes simplex virus thymidine kinase promoter in an orientation-independent manner. In addition, this fragment can confer A23187 inducibility to the neo gene and effectively compete for positive regulatory factors involved in A23187 induction. Sequence analysis of this promoter reveals homology with viral core enhancer sequences, and the apparent organization of direct repeat domains is similar to those observed in viral enhancers.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Attenello J. W., Lee A. S. Regulation of a hybrid gene by glucose and temperature in hamster fibroblasts. Science. 1984 Oct 12;226(4671):187–190. doi: 10.1126/science.6484570. [DOI] [PubMed] [Google Scholar]
- Beck E., Ludwig G., Auerswald E. A., Reiss B., Schaller H. Nucleotide sequence and exact localization of the neomycin phosphotransferase gene from transposon Tn5. Gene. 1982 Oct;19(3):327–336. doi: 10.1016/0378-1119(82)90023-3. [DOI] [PubMed] [Google Scholar]
- Bird A., Taggart M., Frommer M., Miller O. J., Macleod D. A fraction of the mouse genome that is derived from islands of nonmethylated, CpG-rich DNA. Cell. 1985 Jan;40(1):91–99. doi: 10.1016/0092-8674(85)90312-5. [DOI] [PubMed] [Google Scholar]
- Celander D., Haseltine W. A. Tissue-specific transcription preference as a determinant of cell tropism and leukaemogenic potential of murine retroviruses. Nature. 1984 Nov 8;312(5990):159–162. doi: 10.1038/312159a0. [DOI] [PubMed] [Google Scholar]
- Dignam J. D., Martin P. L., Shastry B. S., Roeder R. G. Eukaryotic gene transcription with purified components. Methods Enzymol. 1983;101:582–598. doi: 10.1016/0076-6879(83)01039-3. [DOI] [PubMed] [Google Scholar]
- Dynan W. S., Saffer J. D., Lee W. S., Tjian R. Transcription factor Sp1 recognizes promoter sequences from the monkey genome that are simian virus 40 promoter. Proc Natl Acad Sci U S A. 1985 Aug;82(15):4915–4919. doi: 10.1073/pnas.82.15.4915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dynan W. S., Tjian R. The promoter-specific transcription factor Sp1 binds to upstream sequences in the SV40 early promoter. Cell. 1983 Nov;35(1):79–87. doi: 10.1016/0092-8674(83)90210-6. [DOI] [PubMed] [Google Scholar]
- Gillies S. D., Morrison S. L., Oi V. T., Tonegawa S. A tissue-specific transcription enhancer element is located in the major intron of a rearranged immunoglobulin heavy chain gene. Cell. 1983 Jul;33(3):717–728. doi: 10.1016/0092-8674(83)90014-4. [DOI] [PubMed] [Google Scholar]
- Gluzman Y. SV40-transformed simian cells support the replication of early SV40 mutants. Cell. 1981 Jan;23(1):175–182. doi: 10.1016/0092-8674(81)90282-8. [DOI] [PubMed] [Google Scholar]
- Goodbourn S., Zinn K., Maniatis T. Human beta-interferon gene expression is regulated by an inducible enhancer element. Cell. 1985 Jun;41(2):509–520. doi: 10.1016/s0092-8674(85)80024-6. [DOI] [PubMed] [Google Scholar]
- Gorman C. M., Moffat L. F., Howard B. H. Recombinant genomes which express chloramphenicol acetyltransferase in mammalian cells. Mol Cell Biol. 1982 Sep;2(9):1044–1051. doi: 10.1128/mcb.2.9.1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hearing P., Shenk T. The adenovirus type 5 E1A transcriptional control region contains a duplicated enhancer element. Cell. 1983 Jul;33(3):695–703. doi: 10.1016/0092-8674(83)90012-0. [DOI] [PubMed] [Google Scholar]
- Hirt B. Selective extraction of polyoma DNA from infected mouse cell cultures. J Mol Biol. 1967 Jun 14;26(2):365–369. doi: 10.1016/0022-2836(67)90307-5. [DOI] [PubMed] [Google Scholar]
- Karin M., Haslinger A., Holtgreve H., Richards R. I., Krauter P., Westphal H. M., Beato M. Characterization of DNA sequences through which cadmium and glucocorticoid hormones induce human metallothionein-IIA gene. Nature. 1984 Apr 5;308(5959):513–519. doi: 10.1038/308513a0. [DOI] [PubMed] [Google Scholar]
- Khoury G., Gruss P. Enhancer elements. Cell. 1983 Jun;33(2):313–314. doi: 10.1016/0092-8674(83)90410-5. [DOI] [PubMed] [Google Scholar]
- Labhart P., Reeder R. H. Enhancer-like properties of the 60/81 bp elements in the ribosomal gene spacer of Xenopus laevis. Cell. 1984 May;37(1):285–289. doi: 10.1016/0092-8674(84)90324-6. [DOI] [PubMed] [Google Scholar]
- Lee A. S., Delegeane A. M., Baker V., Chow P. C. Transcriptional regulation of two genes specifically induced by glucose starvation in a hamster mutant fibroblast cell line. J Biol Chem. 1983 Jan 10;258(1):597–603. [PubMed] [Google Scholar]
- Lee A. S. The accumulation of three specific proteins related to glucose-regulated proteins in a temperature-sensitive hamster mutant cell line K12. J Cell Physiol. 1981 Jan;106(1):119–125. doi: 10.1002/jcp.1041060113. [DOI] [PubMed] [Google Scholar]
- Lin A. Y., Lee A. S. Induction of two genes by glucose starvation in hamster fibroblasts. Proc Natl Acad Sci U S A. 1984 Feb;81(4):988–992. doi: 10.1073/pnas.81.4.988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. Sequencing end-labeled DNA with base-specific chemical cleavages. Methods Enzymol. 1980;65(1):499–560. doi: 10.1016/s0076-6879(80)65059-9. [DOI] [PubMed] [Google Scholar]
- McIvor R. S., Goddard J. M., Simonsen C. C., Martin D. W., Jr Expression of a cDNA sequence encoding human purine nucleoside phosphorylase in rodent and human cells. Mol Cell Biol. 1985 Jun;5(6):1349–1357. doi: 10.1128/mcb.5.6.1349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKnight S. L., Kingsbury R. C., Spence A., Smith M. The distal transcription signals of the herpesvirus tk gene share a common hexanucleotide control sequence. Cell. 1984 May;37(1):253–262. doi: 10.1016/0092-8674(84)90321-0. [DOI] [PubMed] [Google Scholar]
- Mercola M., Goverman J., Mirell C., Calame K. Immunoglobulin heavy-chain enhancer requires one or more tissue-specific factors. Science. 1985 Jan 18;227(4684):266–270. doi: 10.1126/science.3917575. [DOI] [PubMed] [Google Scholar]
- Nordheim A., Rich A. Negatively supercoiled simian virus 40 DNA contains Z-DNA segments within transcriptional enhancer sequences. Nature. 1983 Jun 23;303(5919):674–679. doi: 10.1038/303674a0. [DOI] [PubMed] [Google Scholar]
- Parker C. S., Topol J. A Drosophila RNA polymerase II transcription factor binds to the regulatory site of an hsp 70 gene. Cell. 1984 May;37(1):273–283. doi: 10.1016/0092-8674(84)90323-4. [DOI] [PubMed] [Google Scholar]
- Picard D., Schaffner W. A lymphocyte-specific enhancer in the mouse immunoglobulin kappa gene. Nature. 1984 Jan 5;307(5946):80–82. doi: 10.1038/307080a0. [DOI] [PubMed] [Google Scholar]
- Resendez E., Jr, Attenello J. W., Grafsky A., Chang C. S., Lee A. S. Calcium ionophore A23187 induces expression of glucose-regulated genes and their heterologous fusion genes. Mol Cell Biol. 1985 Jun;5(6):1212–1219. doi: 10.1128/mcb.5.6.1212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Southern P. J., Berg P. Transformation of mammalian cells to antibiotic resistance with a bacterial gene under control of the SV40 early region promoter. J Mol Appl Genet. 1982;1(4):327–341. [PubMed] [Google Scholar]
- Treisman R., Maniatis T. Simian virus 40 enhancer increases number of RNA polymerase II molecules on linked DNA. Nature. 1985 May 2;315(6014):73–75. doi: 10.1038/315072a0. [DOI] [PubMed] [Google Scholar]
- Vieira J., Messing J. The pUC plasmids, an M13mp7-derived system for insertion mutagenesis and sequencing with synthetic universal primers. Gene. 1982 Oct;19(3):259–268. doi: 10.1016/0378-1119(82)90015-4. [DOI] [PubMed] [Google Scholar]
- Walker M. D., Edlund T., Boulet A. M., Rutter W. J. Cell-specific expression controlled by the 5'-flanking region of insulin and chymotrypsin genes. Nature. 1983 Dec 8;306(5943):557–561. doi: 10.1038/306557a0. [DOI] [PubMed] [Google Scholar]
- Wang M. L., Lee A. S. Polymerization of vector DNA after transfection into hamster fibroblast cells. Biochem Biophys Res Commun. 1983 Jan 27;110(2):593–601. doi: 10.1016/0006-291x(83)91191-9. [DOI] [PubMed] [Google Scholar]
- Weiher H., König M., Gruss P. Multiple point mutations affecting the simian virus 40 enhancer. Science. 1983 Feb 11;219(4585):626–631. doi: 10.1126/science.6297005. [DOI] [PubMed] [Google Scholar]
- Wolf S. F., Migeon B. R. Clusters of CpG dinucleotides implicated by nuclease hypersensitivity as control elements of housekeeping genes. Nature. 1985 Apr 4;314(6010):467–469. doi: 10.1038/314467a0. [DOI] [PubMed] [Google Scholar]