Abstract
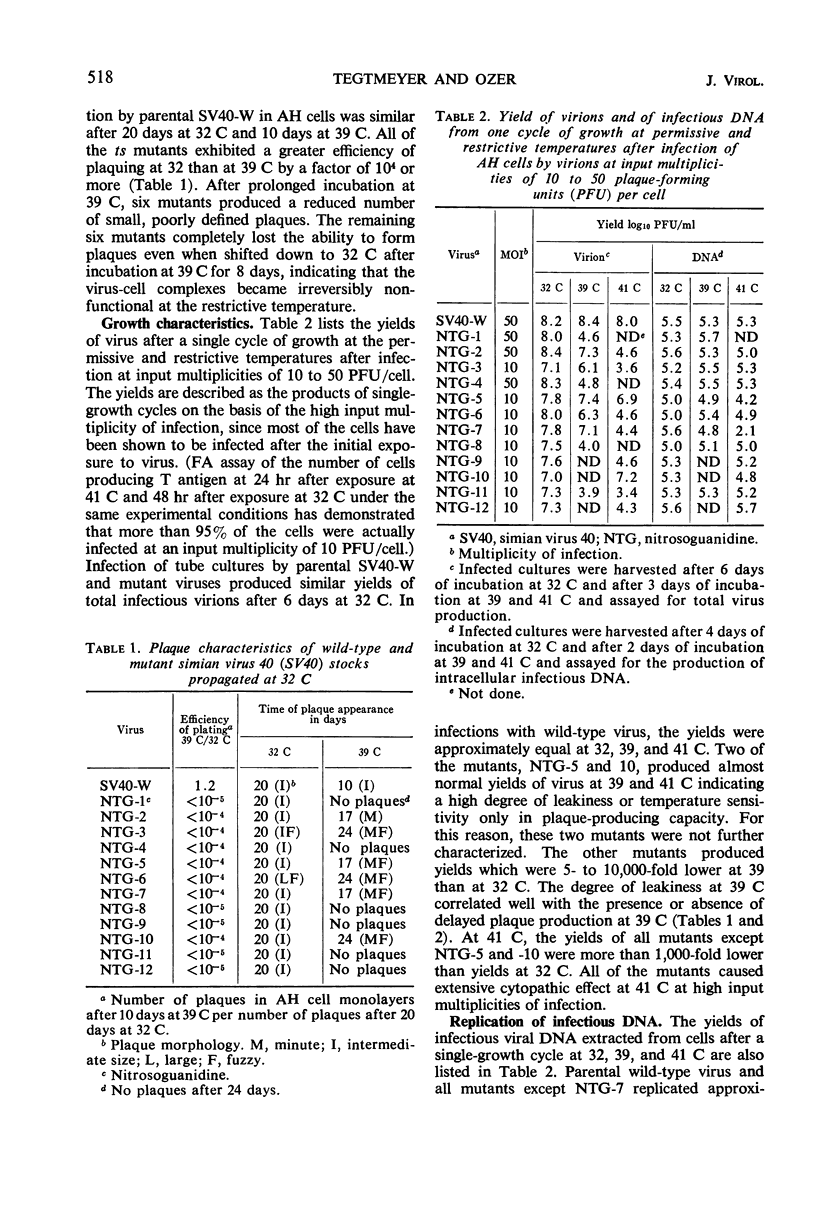
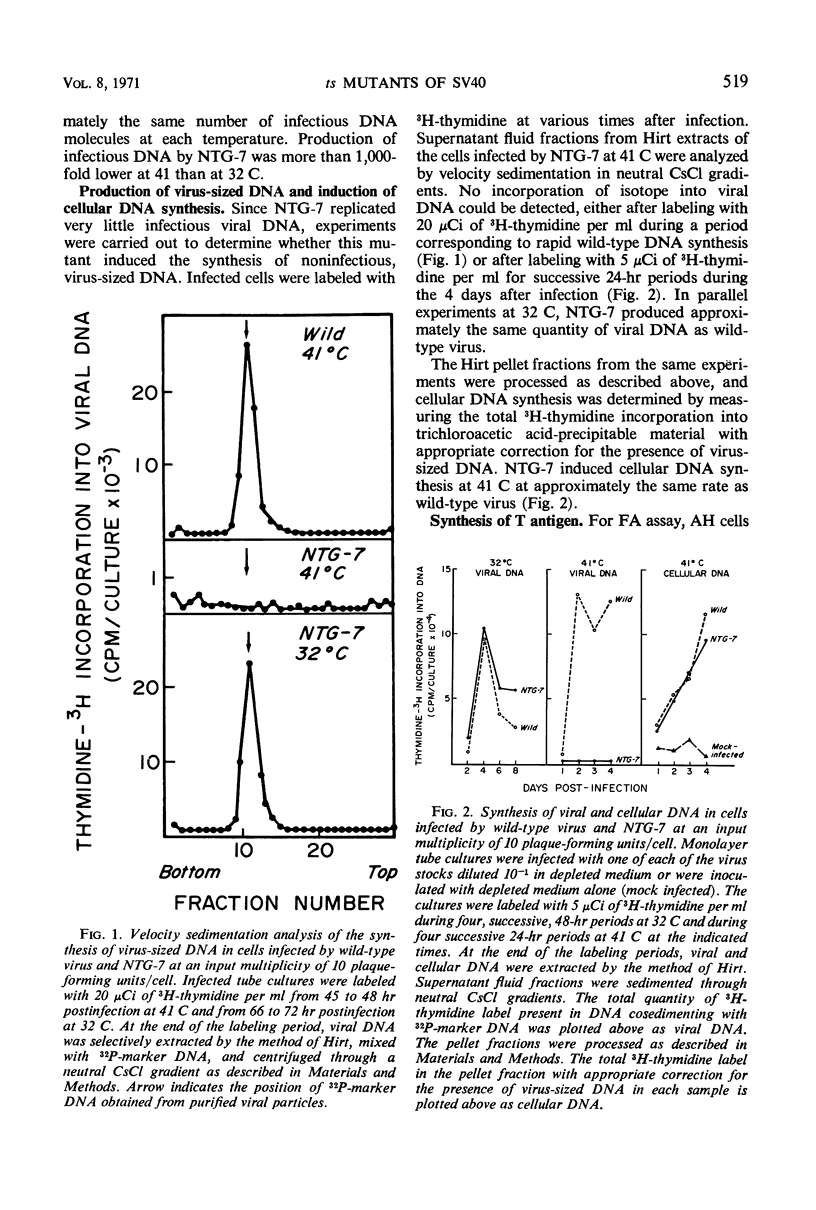
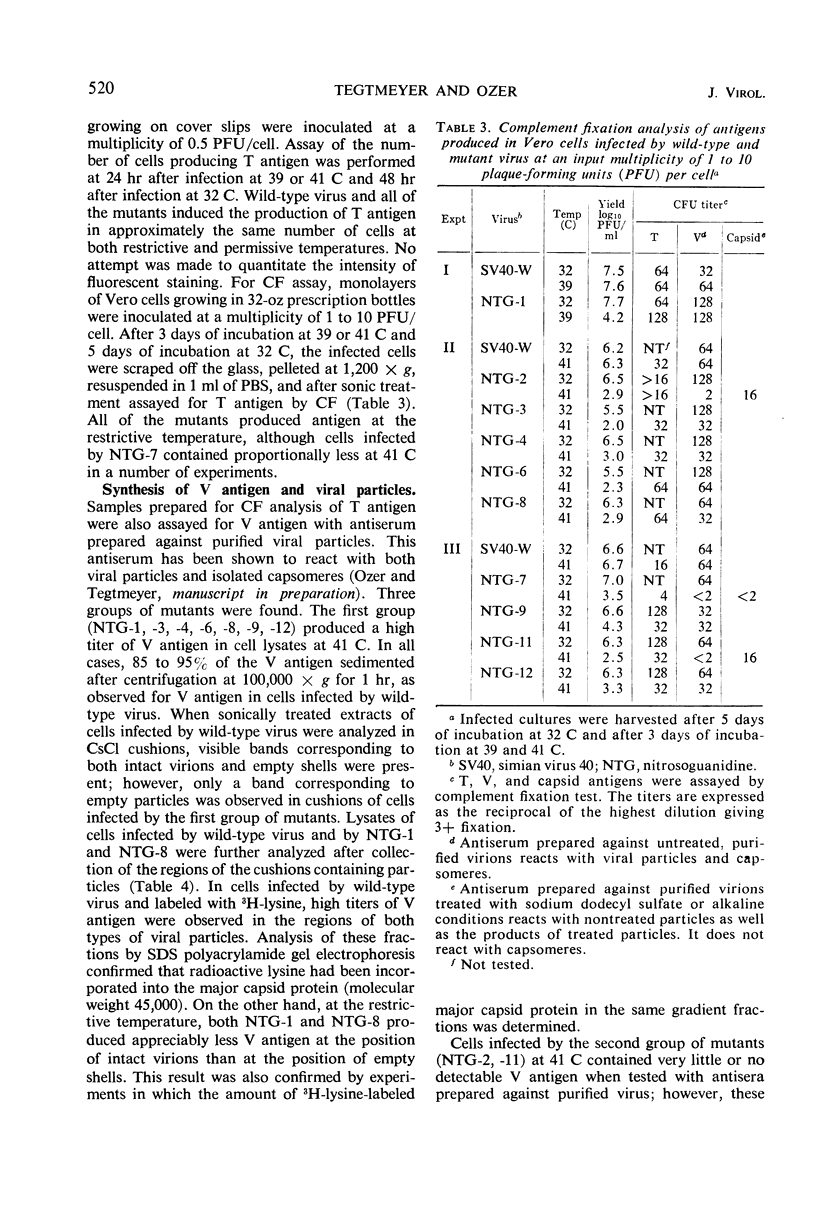
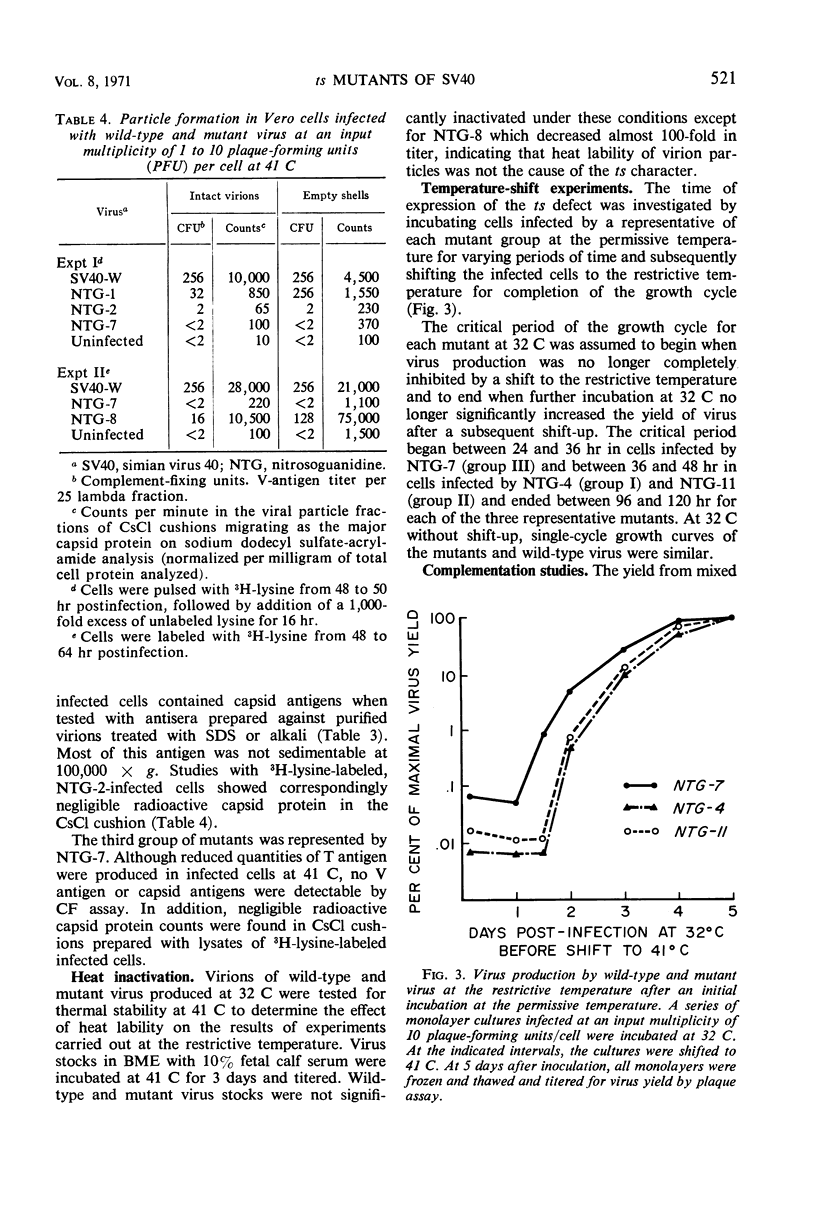
Ten temperature-sensitive mutants of simian virus 40 have been isolated and characterized in permissive cells. The mutants could be divided into three functional groups and two complementation groups. Seven mutants produced T antigen, infectious viral deoxyribonucleic acid (DNA), and structural viral antigen but predominantly the empty shell type of viral particles. Two mutants produced T antigen and infectious viral DNA, but, although viral structural protein(s) could be detected immunologically, no V antigen or viral particles were found. These two functional groups of mutants did not complement each other. A single mutant was defective in the synthesis of viral DNA, viral structural antigens, and viral particles. T antigen could be detected in infected cells by fluorescent antibody but was reduced by complement fixation assay. This mutant stimulated cell DNA synthesis at the restrictive temperature and complemented the other two functional groups of mutants.
Full text
PDF

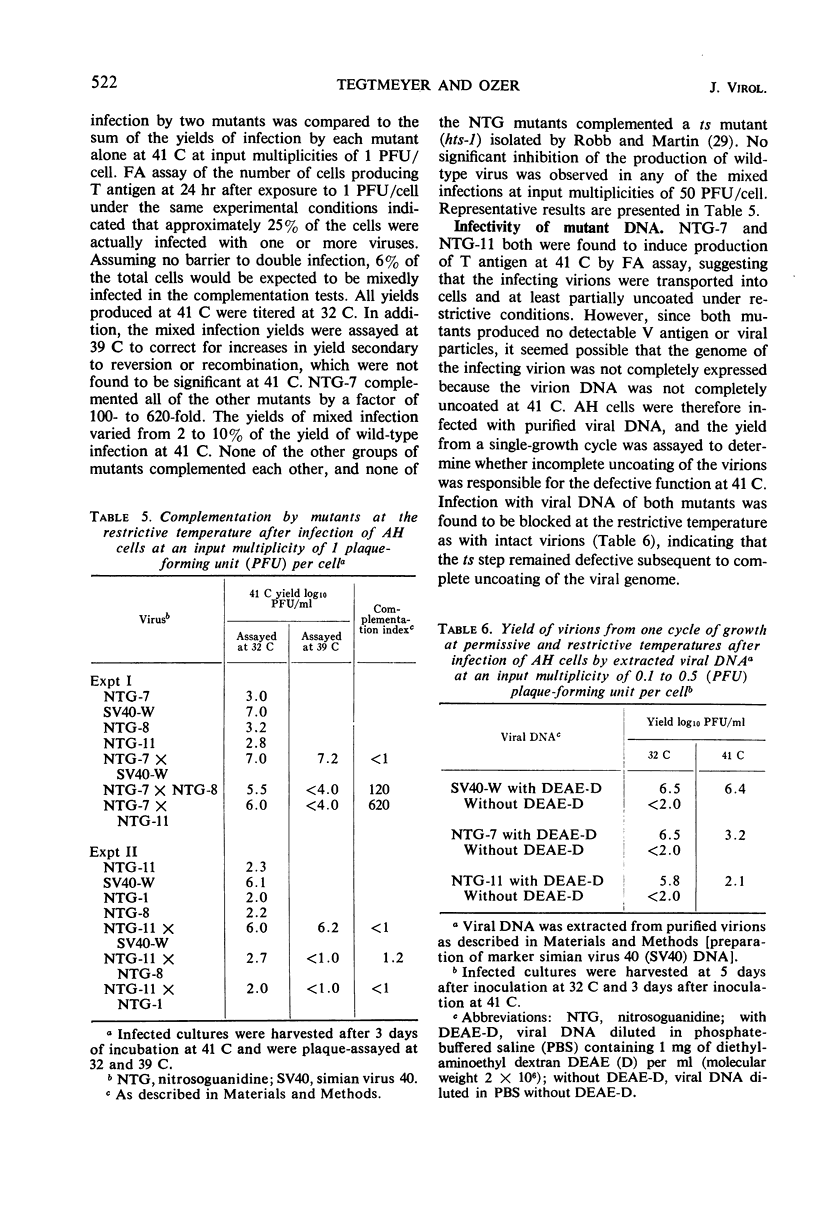
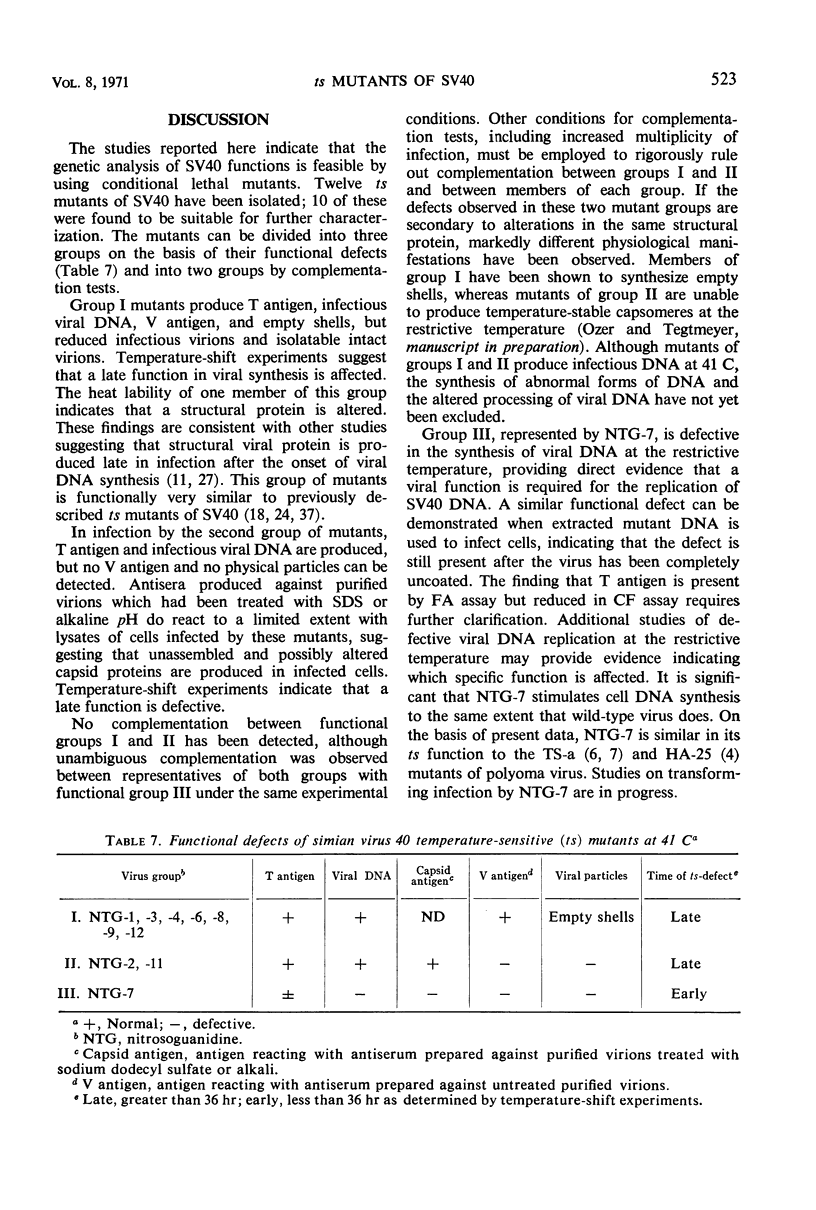

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BLACK P. H., ROWE W. P. SV-40 INDUCED PROLIFERATION OF TISSUE CULTURE CELLS OF RABBIT, MOUSE, AND PORCINE ORIGIN. Proc Soc Exp Biol Med. 1963 Dec;114:721–727. doi: 10.3181/00379727-114-28780. [DOI] [PubMed] [Google Scholar]
- BLACK P. H., ROWE W. P., TURNER H. C., HUEBNER R. J. A SPECIFIC COMPLEMENT-FIXING ANTIGEN PRESENT IN SV40 TUMOR AND TRANSFORMED CELLS. Proc Natl Acad Sci U S A. 1963 Dec;50:1148–1156. doi: 10.1073/pnas.50.6.1148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Black P. H. Transformation of mouse cell line 3T3 by SV40: dose response relationship and correlation with SV40 tumor antigen production. Virology. 1966 Apr;28(4):760–763. doi: 10.1016/0042-6822(66)90262-5. [DOI] [PubMed] [Google Scholar]
- Di Mayorca G., Callender J., Marin G., Giordano R. Temperature-sensitive mutants of polyoma virus. Virology. 1969 May;38(1):126–133. doi: 10.1016/0042-6822(69)90134-2. [DOI] [PubMed] [Google Scholar]
- Earley E., Peralta P. H., Johnson K. M. A plaque neutralization method for arboviruses. Proc Soc Exp Biol Med. 1967 Jul;125(3):741–747. doi: 10.3181/00379727-125-32194. [DOI] [PubMed] [Google Scholar]
- Eckhart W. Complementation and transformation by temperature-sensitive mutants of polyoma virus. Virology. 1969 May;38(1):120–125. doi: 10.1016/0042-6822(69)90133-0. [DOI] [PubMed] [Google Scholar]
- Fried M. Characterization of a temperature-sensitive mutant of polyoma virus. Virology. 1970 Mar;40(3):605–617. doi: 10.1016/0042-6822(70)90205-9. [DOI] [PubMed] [Google Scholar]
- Friedmann T., Haas M. Rapid concentration and purification of polyoma virus and SV40 with polyethylene glycol. Virology. 1970 Sep;42(1):248–250. doi: 10.1016/0042-6822(70)90263-1. [DOI] [PubMed] [Google Scholar]
- GILDEN R. V., CARP R. I., TAGUCHI F., DEFEND V. THE NATURE AND LOCALIZATION OF THE SV 40-INDUCED COMPLEMENT-FIXING ANTIGEN. Proc Natl Acad Sci U S A. 1965 Mar;53:684–692. doi: 10.1073/pnas.53.3.684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GUENALP A. GROWTH AND CYTOPATHIC EFFECT OF RUBELLA VIRUS IN A LINE OF GREEN MONKEY KIDNEY CELLS. Proc Soc Exp Biol Med. 1965 Jan;118:85–90. [PubMed] [Google Scholar]
- Gerber P. Studies on the transfer of subviral infectivity from SV40-induced hamster tumor cells to indicator cells. Virology. 1966 Apr;28(4):501–509. doi: 10.1016/0042-6822(66)90234-0. [DOI] [PubMed] [Google Scholar]
- Gershon D., Sachs L., Winocour E. The induction of cellular DNA synthesis by simian virus 40 in contact-inhibited and in x-irradiated cells. Proc Natl Acad Sci U S A. 1966 Sep;56(3):918–925. doi: 10.1073/pnas.56.3.918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HOPPS H. E., BERNHEIM B. C., NISALAK A., TJIO J. H., SMADEL J. E. BIOLOGIC CHARACTERISTICS OF A CONTINUOUS KIDNEY CELL LINE DERIVED FROM THE AFRICAN GREEN MONKEY. J Immunol. 1963 Sep;91:416–424. [PubMed] [Google Scholar]
- Hatanaka M., Dulbecco R. Induction of DNA synthesis by SV40. Proc Natl Acad Sci U S A. 1966 Aug;56(2):736–740. doi: 10.1073/pnas.56.2.736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henry P., Black P. H., Oxman M. N., Weissman S. M. Stimulation of DNA synthesis in mouse cell line 3T3 by Simian virus 40. Proc Natl Acad Sci U S A. 1966 Oct;56(4):1170–1176. doi: 10.1073/pnas.56.4.1170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirt B. Selective extraction of polyoma DNA from infected mouse cell cultures. J Mol Biol. 1967 Jun 14;26(2):365–369. doi: 10.1016/0022-2836(67)90307-5. [DOI] [PubMed] [Google Scholar]
- Kit S., De Torres R. A., Dubbs D. R., Salvi M. L. Induction of cellular deoxyribonuleic acid synthesis by simian virus 40. J Virol. 1967 Aug;1(4):738–746. doi: 10.1128/jvi.1.4.738-746.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kit S., Tokuno S., Nakajima K., Trkula D., Dubbs D. R. Temperature-sensitive simian virus 40 mutant defective in a late function. J Virol. 1970 Sep;6(3):286–294. doi: 10.1128/jvi.6.3.286-294.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis A. M., Jr, Rowe W. P. Studies on nondefective adenovirus-simian virus 40 hybrid viruses. I. A newly characterized simian virus 40 antigen induced by the Ad2+ND 1 virus. J Virol. 1971 Feb;7(2):189–197. doi: 10.1128/jvi.7.2.189-197.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MAYOR H. D., STINEBAUGH S. E., JAMISON R. M., JORDAN L. E., MELNICK J. L. Immunofluorescent, cytochemical, and microcytological studies on the growth of the simian vacuolating virus (SV-40) in tissue culture. Exp Mol Pathol. 1962 Oct;1:397–416. doi: 10.1016/0014-4800(62)90033-3. [DOI] [PubMed] [Google Scholar]
- McCutchan J. H., Pagano J. S. Enchancement of the infectivity of simian virus 40 deoxyribonucleic acid with diethylaminoethyl-dextran. J Natl Cancer Inst. 1968 Aug;41(2):351–357. [PubMed] [Google Scholar]
- Oxman M. N., Baron S., Black P. H., Takemoto K. K., Habel K., Rowe W. P. The effect of interferon on SV-40 T antigen production in SV-40-transformed cells. Virology. 1967 May;32(1):122–127. doi: 10.1016/0042-6822(67)90260-7. [DOI] [PubMed] [Google Scholar]
- Ozer H. L., Takemoto K. K. Site of host restriction of simian virus 40 mutants in an established African green monkey kidney cell line. J Virol. 1969 Oct;4(4):408–415. doi: 10.1128/jvi.4.4.408-415.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PATTERSON M. S., GREENE R. C. MEASUREMENT OF LOW ENERGY BETA-EMITTERS IN AQUEOUS SOLUTION BY LIQUID SCINTILLATION COUNTING OF EMULSIONS. Anal Chem. 1965 Jun;37:854–857. doi: 10.1021/ac60226a017. [DOI] [PubMed] [Google Scholar]
- POPE J. H., ROWE W. P. DETECTION OF SPECIFIC ANTIGEN IN SV40-TRANSFORMED CELLS BY IMMUNOFLUORESCENCE. J Exp Med. 1964 Aug 1;120:121–128. doi: 10.1084/jem.120.2.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RAPP F., KITAHARA T., BUTEL J. S., MELNICK J. L. SYNTHESIS OF SV40 TUMOR ANTIGEN DURING REPLICATION OF SIMIAN PAPOVAVIRUS (SV40). Proc Natl Acad Sci U S A. 1964 Nov;52:1138–1142. doi: 10.1073/pnas.52.5.1138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritzi E., Levine A. J. Deoxyribonucleic acid replication in simian virus 40-infected cells. 3. Comparison of simian virus 40 lytic infection in three different monkey kidney cell lines. J Virol. 1970 Jun;5(6):686–692. doi: 10.1128/jvi.5.6.686-692.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robb J. A., Martin R. G. Genetic analysis of simian virus 40. I. Description of microtitration and replica-plating techniques for virus. Virology. 1970 Aug;41(4):751–760. doi: 10.1016/0042-6822(70)90439-3. [DOI] [PubMed] [Google Scholar]
- SABIN A. B., KOCH M. A. SOURCE OF GENETIC INFORMATION FOR SPECIFIC COMPLEMENT-FIXING ANTIGENS IN SV40 VIRUS-INDUCED TUMORS. Proc Natl Acad Sci U S A. 1964 Nov;52:1131–1138. doi: 10.1073/pnas.52.5.1131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SABIN A. B., SHEIN H. M., KOCH M. A., ENDERS J. F. SPECIFIC COMPLEMENT-FIXING TUMOR ANTIGENS IN HUMAN CELLS MORPHOLOGICALLY TRANSFORMED BY SV40 VIRUS. Proc Natl Acad Sci U S A. 1964 Dec;52:1316–1318. doi: 10.1073/pnas.52.6.1316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SHEIN H. M., ENDERS J. F., LEVINTHAL J. D. Transformation induced by simian virus 40 in human renal cell cultures. II. Cell-virus relationships. Proc Natl Acad Sci U S A. 1962 Aug;48:1350–1357. doi: 10.1073/pnas.48.8.1350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SHEIN H. M., ENDERS J. F. Transformation induced by simian virus 40 in human renal cell cultures. I. Morphology and growth characteristics. Proc Natl Acad Sci U S A. 1962 Jul 15;48:1164–1172. doi: 10.1073/pnas.48.7.1164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SWEET B. H., HILLEMAN M. R. The vacuolating virus, S.V. 40. Proc Soc Exp Biol Med. 1960 Nov;105:420–427. doi: 10.3181/00379727-105-26128. [DOI] [PubMed] [Google Scholar]
- Sambrook J., Westphal H., Srinivasan P. R., Dulbecco R. The integrated state of viral DNA in SV40-transformed cells. Proc Natl Acad Sci U S A. 1968 Aug;60(4):1288–1295. doi: 10.1073/pnas.60.4.1288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schell K., Maryak J. Susceptibility of rabbit kidney cells of various passage levels to infection with SV-40 virus. Arch Gesamte Virusforsch. 1966;19(4):403–414. doi: 10.1007/BF01250609. [DOI] [PubMed] [Google Scholar]
- Takemoto K. K., Martin M. A. SV40 thermosensitive mutant: synthesis of viral DNA and virus-induced proteins at nonpermissive temperature. Virology. 1970 Dec;42(4):938–945. doi: 10.1016/0042-6822(70)90342-9. [DOI] [PubMed] [Google Scholar]
- Tegtmeyer P., Dohan C., Jr, Reznikoff C. Inactivating and mutagenic effects of nitrosoguanidine on simian virus 40. Proc Natl Acad Sci U S A. 1970 Jul;66(3):745–752. doi: 10.1073/pnas.66.3.745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Todaro G. J., Green H. High frequency of SV40 transformation of mouse cell line 3T3. Virology. 1966 Apr;28(4):756–759. doi: 10.1016/0042-6822(66)90261-3. [DOI] [PubMed] [Google Scholar]


