Abstract
Cell-free translation of poly(A)-containing RNA from the JY lymphoblastoid cell line followed by immunoprecipitation has indicated the presence of mRNAs for both the heavy and light chains of HLA-A and HLA-B antigens in these preparations. Both chains are synthesized with an NH2-terminal extension, approximately 20 residues in length for the light chain, and 20 or 24 residues for the heavy chains. The precursors can be processed by dog pancreatic microsomes to products similar to those obtained in vivo. Immunoprecipitation of the cell-free products has been employed as an assay for partial purification of the mRNAs. Investigation of the Daudi cell line, which cannot synthesize the small subunit, beta 2-microglobin, has indicated that the heavy chains of HLA-A and HLA-B antigens are synthesized intracellularly in vivo and can also be translated from their cognate RNAs in vitro. The implications of these findings for biosynthesis of membrane proteins in general and multimeric membrane proteins in particular, as well as the role of beta 2-microglobulin in expression of HLA-A and HLA-B antigens, are discussed.
Full text
PDF

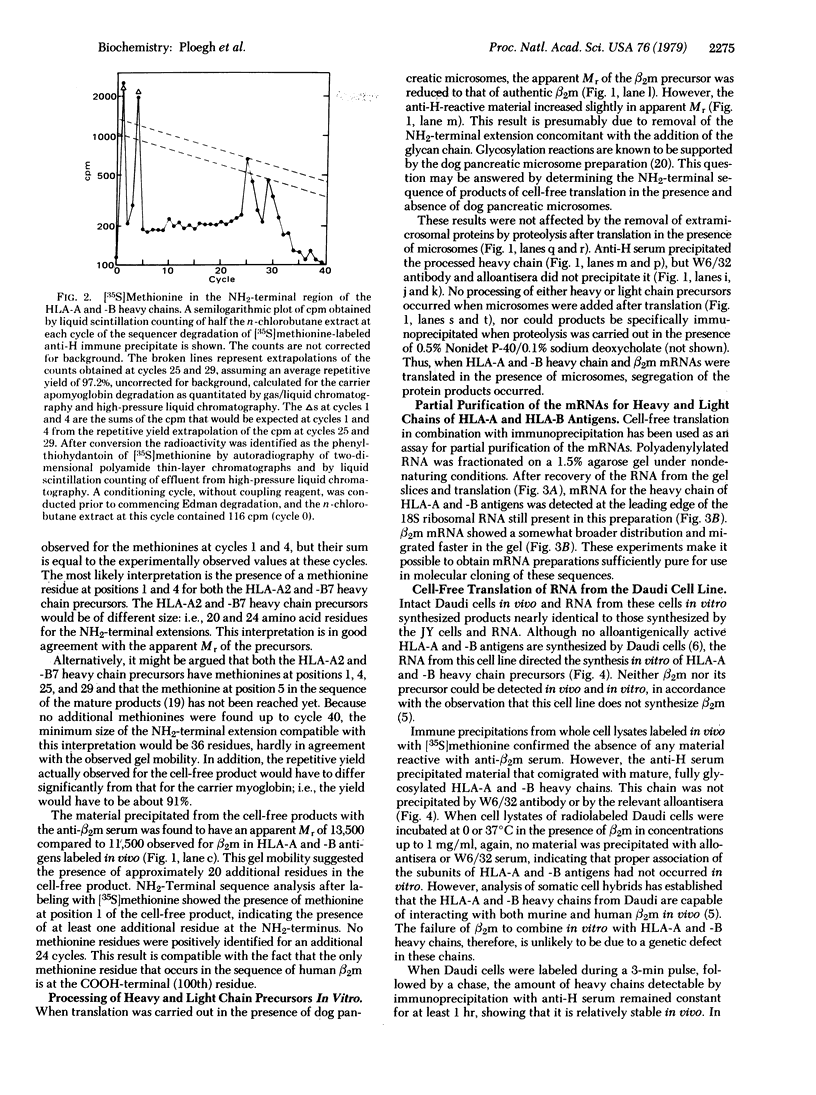


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arce-Gomez B., Jones E. A., Barnstable C. J., Solomon E., Bodmer W. F. The genetic control of HLA-A and B antigens in somatic cell hybrids: requirement for beta2 microglobulin. Tissue Antigens. 1978 Feb;11(2):96–112. doi: 10.1111/j.1399-0039.1978.tb01233.x. [DOI] [PubMed] [Google Scholar]
- Barnstable C. J., Bodmer W. F., Brown G., Galfre G., Milstein C., Williams A. F., Ziegler A. Production of monoclonal antibodies to group A erythrocytes, HLA and other human cell surface antigens-new tools for genetic analysis. Cell. 1978 May;14(1):9–20. doi: 10.1016/0092-8674(78)90296-9. [DOI] [PubMed] [Google Scholar]
- Blobel G., Dobberstein B. Transfer of proteins across membranes. I. Presence of proteolytically processed and unprocessed nascent immunoglobulin light chains on membrane-bound ribosomes of murine myeloma. J Cell Biol. 1975 Dec;67(3):835–851. doi: 10.1083/jcb.67.3.835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonner W. M., Laskey R. A. A film detection method for tritium-labelled proteins and nucleic acids in polyacrylamide gels. Eur J Biochem. 1974 Jul 1;46(1):83–88. doi: 10.1111/j.1432-1033.1974.tb03599.x. [DOI] [PubMed] [Google Scholar]
- Brauer A. W., Margolies M. N., Haber E. The application of 0.1 M quadrol to the microsequence of proteins and the sequence of tryptic peptides. Biochemistry. 1975 Jul;14(13):3029–3035. doi: 10.1021/bi00684a036. [DOI] [PubMed] [Google Scholar]
- Burstein Y., Schechter I. Primary structures of N-terminal extra peptide segments linked to the variable and constant regions of immunoglobulin light chain precursors: implications on the organization and controlled expression of immunoglobulin genes. Biochemistry. 1978 Jun 13;17(12):2392–2400. doi: 10.1021/bi00605a022. [DOI] [PubMed] [Google Scholar]
- Cleveland D. W., Kirschner M. W., Cowan N. J. Isolation of separate mRNAs for alpha- and beta-tubulin and characterization of the corresponding in vitro translation products. Cell. 1978 Nov;15(3):1021–1031. doi: 10.1016/0092-8674(78)90286-6. [DOI] [PubMed] [Google Scholar]
- Devillers-Thiery A., Kindt T., Scheele G., Blobel G. Homology in amino-terminal sequence of precursors to pancreatic secretory proteins. Proc Natl Acad Sci U S A. 1975 Dec;72(12):5016–5020. doi: 10.1073/pnas.72.12.5016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodfellow P. N., Jones E. A., Van Heyningen V., Solomon E., Bobrow M., Miggiano V., Bodmer W. F. The beta2-microglobulin gene is on chromosome 15 and not in the HL-A region. Nature. 1975 Mar 20;254(5497):267–269. doi: 10.1038/254267a0. [DOI] [PubMed] [Google Scholar]
- Kemper B., Habener J. F., Ernst M. D., Potts J. T., Jr, Rich A. Pre-proparathyroid hormone: analysis of radioactive tryptic peptides and amino acid sequence. Biochemistry. 1976 Jan 13;15(1):15–19. doi: 10.1021/bi00646a003. [DOI] [PubMed] [Google Scholar]
- Kessler S. W. Cell membrane antigen isolation with the staphylococcal protein A-antibody adsorbent. J Immunol. 1976 Nov;117(5 Pt 1):1482–1490. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lingappa V. R., Katz F. N., Lodish H. F., Blobel G. A signal sequence for the insertion of a transmembrane glycoprotein. Similarities to the signals of secretory proteins in primary structure and function. J Biol Chem. 1978 Dec 25;253(24):8667–8670. [PubMed] [Google Scholar]
- Ostberg L., Rask L., Nilsson K., Peterson P. A. Independent expression of the two HL-A antigen polypeptide chains. Eur J Immunol. 1976 Jul;5(7):462–468. doi: 10.1002/eji.1830050707. [DOI] [PubMed] [Google Scholar]
- Parham P., Alpert B. N., Orr H. T., Strominger J. L. Carbohydrate moiety of HLA antigens. Antigenic properties and amino acid sequences around the site of glycosylation. J Biol Chem. 1977 Nov 10;252(21):7555–7567. [PubMed] [Google Scholar]
- Pelham H. R., Jackson R. J. An efficient mRNA-dependent translation system from reticulocyte lysates. Eur J Biochem. 1976 Aug 1;67(1):247–256. doi: 10.1111/j.1432-1033.1976.tb10656.x. [DOI] [PubMed] [Google Scholar]
- Rothman J. E., Katz F. N., Lodish H. F. Glycosylation of a membrane protein is restricted to the growing polypeptide chain but is not necessary for insertion as a transmembrane protein. Cell. 1978 Dec;15(4):1447–1454. doi: 10.1016/0092-8674(78)90068-5. [DOI] [PubMed] [Google Scholar]
- Shields D., Blobel G. Efficient cleavage and segregation of nascent presecretory proteins in a reticulocyte lysate supplemented with microsomal membranes. J Biol Chem. 1978 Jun 10;253(11):3753–3756. [PubMed] [Google Scholar]
- Springer T. A., Strominger J. L. Detergent-soluble HLA antigens contain a hydrophilic region at the COOH-terminus and a penultimate hydrophobic region. Proc Natl Acad Sci U S A. 1976 Jul;73(7):2481–2485. doi: 10.1073/pnas.73.7.2481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strominger J. L., Cresswell P., Grey H., Humphreys R. E., Mann D., McCuneJ, Parham P., Robb R., Sanderson A. R., Springer T. A. The immunoglobulin-like structure of human histocompatibility antigens. Transplant Rev. 1974;21(0):126–143. doi: 10.1111/j.1600-065x.1974.tb01549.x. [DOI] [PubMed] [Google Scholar]
- Strominger J. L., Mann D. L., Parham P., Robb R., Springer T., Terhorst C. Structure of HL-A A and B antigens isolated from cultured human lymphocytes. Cold Spring Harb Symp Quant Biol. 1977;41(Pt 1):323–329. doi: 10.1101/sqb.1977.041.01.038. [DOI] [PubMed] [Google Scholar]
- Tanigaki N., Nakamuro K., Natori T., Kreiter V. P., Pressman D. Common antigenic structures of HL-A antigens. V. An antigenic determinant characteristic of a 33,000-dalton fragment of HL-A molecules. Transplantation. 1974 Jul;18(1):74–78. doi: 10.1097/00007890-197407000-00012. [DOI] [PubMed] [Google Scholar]
- Terhorst C., Parham P., Mann D. L., Strominger J. L. Structure of HLA antigens: amino-acid and carbohydrate compositions and NH2-terminal sequences of four antigen preparations. Proc Natl Acad Sci U S A. 1976 Mar;73(3):910–914. doi: 10.1073/pnas.73.3.910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tkacz J. S., Lampen O. Tunicamycin inhibition of polyisoprenyl N-acetylglucosaminyl pyrophosphate formation in calf-liver microsomes. Biochem Biophys Res Commun. 1975 Jul 8;65(1):248–257. doi: 10.1016/s0006-291x(75)80086-6. [DOI] [PubMed] [Google Scholar]





