Abstract
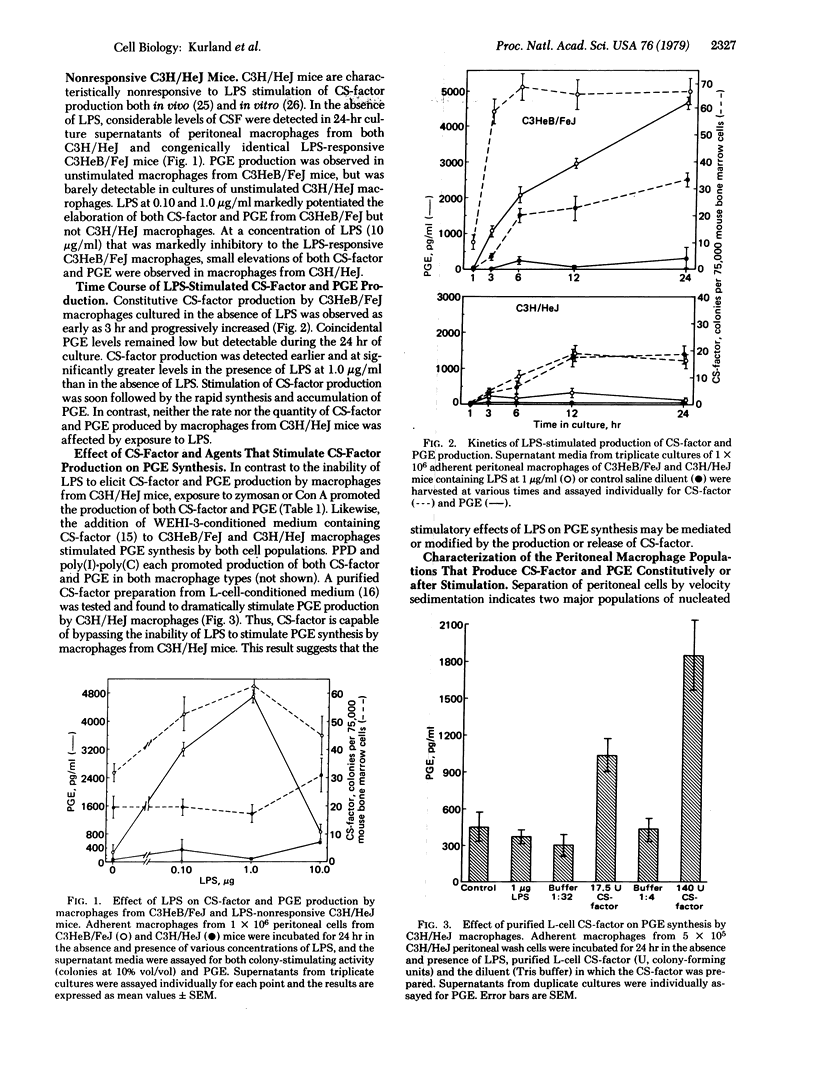
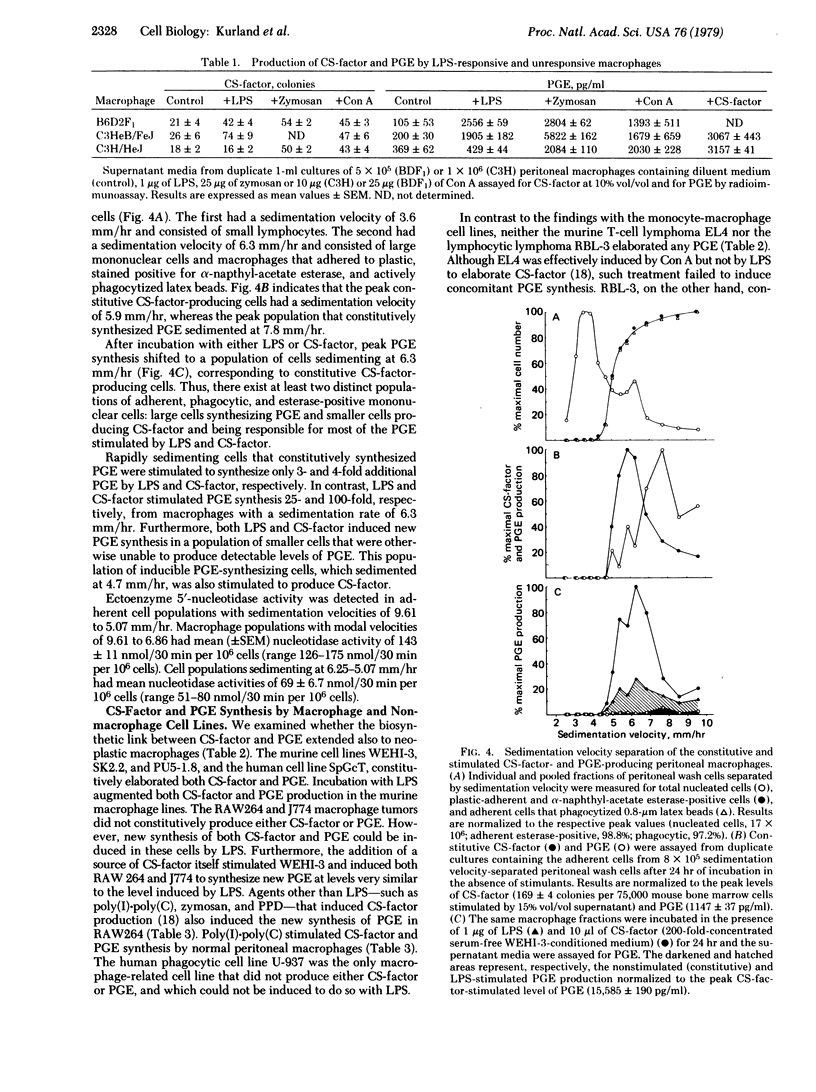
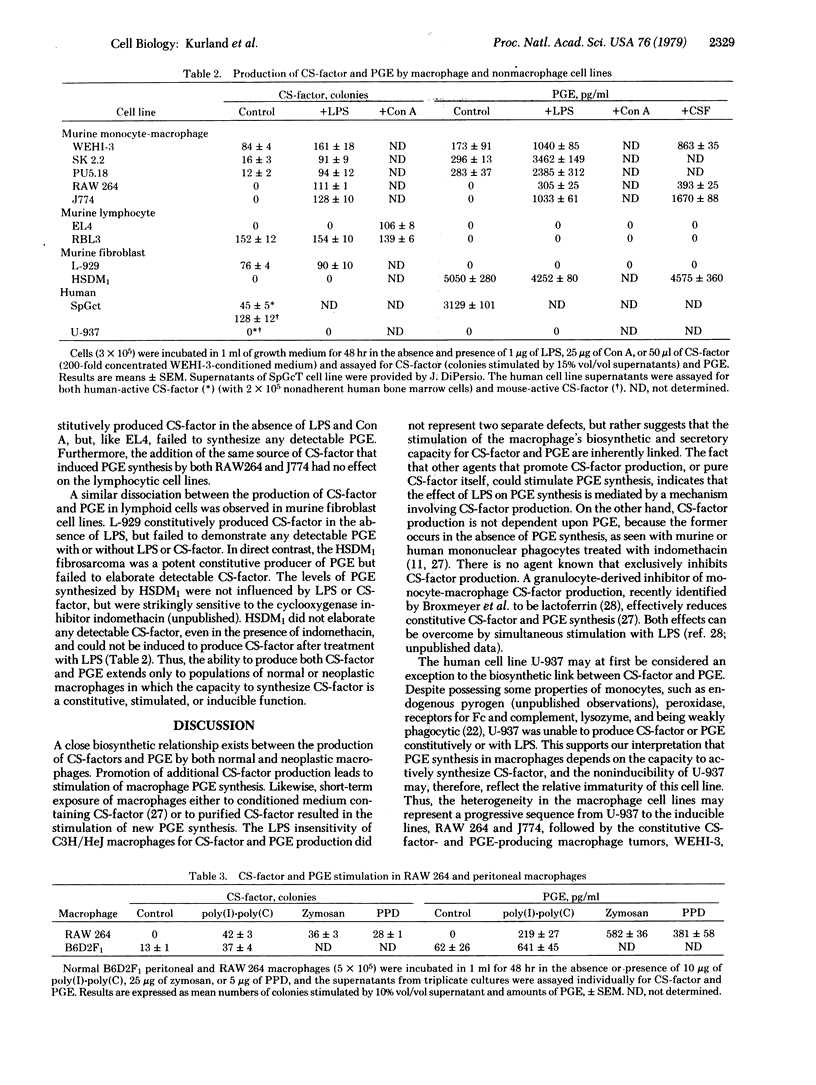
The biosynthesis of prostaglandin E (PGE) by normal and neoplastic macrophages is intrinsically linked to their synthesis of, and exposure to, myeloid colony-stimulating factors (CS-factors). The defect in responsiveness to endotoxin lipopolysaccharide (LPS) by macrophages from C3H/HeJ mice extends equally to the synthesis of CS-factor and PGE. However, C3H/HeJ macrophages can be stimulated to synthesize PGE by treatment with agents other than LPS [zymosan, tuberculin purified protein derivative, concanavalin A, poly(I).poly(C)], which also stimulate CS-factor production, or by the addition of various preparations of soluble CS-factor. In peritoneal wash preparations, constitutive PGE synthesis occurred in rapidly sedimenting macrophage cells, whereas constitutive CS-factor production and inducible PGE synthesis occurred in slower sedimenting adherent cells. A similar functional heterogeneity in CS-factor and PGE production was found in neoplastic macrophagae cell lines. The association of elevated CS-factor levels and PGE synthesis by macrophages suggests a role for CS-factor in many of the physiological responses heretofore associated with elevated tissue levels of the E type prostaglandins.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Apte R. N., Pluznik D. H. Genetic control of lipopolysaccharide induced generation of serum colony stimulating factor and proliferation of splenic granulocyte/macrophage precursor cells. J Cell Physiol. 1976 Oct;89(2):313–323. doi: 10.1002/jcp.1040890214. [DOI] [PubMed] [Google Scholar]
- Avruch J., Wallach D. F. Preparation and properties of plasma membrane and endoplasmic reticulum fragments from isolated rat fat cells. Biochim Biophys Acta. 1971 Apr 13;233(2):334–347. doi: 10.1016/0005-2736(71)90331-2. [DOI] [PubMed] [Google Scholar]
- Broxmeyer H. E. Inhibition in vivo of mouse granulopoiesis by cell-free activity derived from human polymorphonuclear neutrophils. Blood. 1978 May;51(5):889–901. [PubMed] [Google Scholar]
- Broxmeyer H. E., Smithyman A., Eger R. R., Meyers P. A., de Sousa M. Identification of lactoferrin as the granulocyte-derived inhibitor of colony-stimulating activity production. J Exp Med. 1978 Oct 1;148(4):1052–1067. doi: 10.1084/jem.148.4.1052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgess A. W., Camakaris J., Metcalf D. Purification and properties of colony-stimulating factor from mouse lung-conditioned medium. J Biol Chem. 1977 Mar 25;252(6):1998–2003. [PubMed] [Google Scholar]
- Chervenick P. A., LoBuglio A. F. Human blood monocytes: stimulators of granulocyte and mononuclear colony formation in vitro. Science. 1972 Oct 13;178(4057):164–166. doi: 10.1126/science.178.4057.164. [DOI] [PubMed] [Google Scholar]
- Cline M. J., Rothman B., Golde D. W. Effect of endotoxin on the production of colony-stimulating factor by human monocytes and macrophages. J Cell Physiol. 1974 Oct;84(2):193–196. doi: 10.1002/jcp.1040840205. [DOI] [PubMed] [Google Scholar]
- Di Persio J. F., Brennan J. K., Lichtman M. A., Speiser B. L. Human cell lines that elaborate colon-stimulating activity for the marrow cells of man and other species. Blood. 1978 Mar;51(3):507–519. [PubMed] [Google Scholar]
- Eaves A. C., Bruce W. R. In vitro production of colony-stimulating activity. I. Exposure of mouse peritoneal cells to endotoxin. Cell Tissue Kinet. 1974 Jan;7(1):19–30. doi: 10.1111/j.1365-2184.1974.tb00395.x. [DOI] [PubMed] [Google Scholar]
- Edelson P. J., Cohn Z. A. 5'-Nucleotidase activity of mouse peritoneal macrophages. I. Synthesis and degradation in resident and inflammatory populations. J Exp Med. 1976 Dec 1;144(6):1581–1595. doi: 10.1084/jem.144.6.1581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golde D. W., Cline M. J. Identification of the colony-stimulating cell in human peripheral blood. J Clin Invest. 1972 Nov;51(11):2981–2983. doi: 10.1172/JCI107124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Humes J. L., Bonney R. J., Pelus L., Dahlgren M. E., Sadowski S. J., Kuehl F. A., Jr, Davies P. Macrophages synthesis and release prostaglandins in response to inflammatory stimuli. Nature. 1977 Sep 8;269(5624):149–151. doi: 10.1038/269149a0. [DOI] [PubMed] [Google Scholar]
- Kurland J. I., Bockman R. S., Broxmeyer H. E., Moore M. A. Limitation of excessive myelopoiesis by the intrinsic modulation of macrophage-derived prostaglandin E. Science. 1978 Feb 3;199(4328):552–555. doi: 10.1126/science.304600. [DOI] [PubMed] [Google Scholar]
- Kurland J. I., Bockman R. Prostaglandin E production by human blood monocytes and mouse peritoneal macrophages. J Exp Med. 1978 Mar 1;147(3):952–957. doi: 10.1084/jem.147.3.952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurland J. I., Broxmeyer H. E., Pelus L. M., Bockman R. S., Moore M. A. Role for monocyte-macrophage-derived colony-stimulating factor and prostaglandin E in the positive and negative feedback control of myeloid stem cell proliferation. Blood. 1978 Aug;52(2):388–407. [PubMed] [Google Scholar]
- Kurland J. I., Kincade P. W., Moore M. A. Regulation of B-lymphocyte clonal proliferation by stimulatory and inhibitory macrophage-derived factors. J Exp Med. 1977 Nov 1;146(5):1420–1435. doi: 10.1084/jem.146.5.1420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin H., Stewart C. C. Colony formation by mouse peritoneal exudate cells in vitro. Nat New Biol. 1973 Jun 6;243(127):176–177. doi: 10.1038/newbio243176a0. [DOI] [PubMed] [Google Scholar]
- Miller R. G., Phillips R. A. Separation of cells by velocity sedimentation. J Cell Physiol. 1969 Jun;73(3):191–201. doi: 10.1002/jcp.1040730305. [DOI] [PubMed] [Google Scholar]
- Moore M. A., Dexter T. M. Stem cell regulation in continuous hematopoietic cell culture. Transplant Proc. 1978 Mar;10(1):83–90. [PubMed] [Google Scholar]
- Moore M. A., Williams N. Physical separation of colony stimulating cells from in vitro colony forming cells in hemopoietic tissue. J Cell Physiol. 1972 Oct;80(2):195–206. doi: 10.1002/jcp.1040800206. [DOI] [PubMed] [Google Scholar]
- Quesenberry P., Morley A., Stohlman F., Jr, Rickard K., Howard D., Smith M. Effect of endotoxin on granulopoiesis and colony-stimulating factor. N Engl J Med. 1972 Feb 3;286(5):227–232. doi: 10.1056/NEJM197202032860502. [DOI] [PubMed] [Google Scholar]
- Ralph P., Broxmeyer H. E., Moore M. A., Nakoinz I. Induction of myeloid colony-stimulating activity in murine monocyte tumor cell lines by macrophage activators and in a T-cell line by concanavalin A. Cancer Res. 1978 May;38(5):1414–1419. [PubMed] [Google Scholar]
- Ralph P., Nakoinz I. Antibody-dependent killing of erythrocyte and tumor targets by macrophage-related cell lines: enhancement by PPD and LPS. J Immunol. 1977 Sep;119(3):950–954. [PubMed] [Google Scholar]
- Russo M., Lutton J. D. Decreased in vivo and in vitro colony stimulating activity responses to bacterial lipopolysaccharide in C3H/HeJ mice. J Cell Physiol. 1977 Aug;92(2):303–307. doi: 10.1002/jcp.1040920219. [DOI] [PubMed] [Google Scholar]
- Stanley E. R., Cifone M., Heard P. M., Defendi V. Factors regulating macrophage production and growth: identity of colony-stimulating factor and macrophage growth factor. J Exp Med. 1976 Mar 1;143(3):631–647. doi: 10.1084/jem.143.3.631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sundström C., Nilsson K. Establishment and characterization of a human histiocytic lymphoma cell line (U-937). Int J Cancer. 1976 May 15;17(5):565–577. doi: 10.1002/ijc.2910170504. [DOI] [PubMed] [Google Scholar]
- Tashjian A. H., Jr, Voelkel E. F., Levine L., Goldhaber P. Evidence that the bone resorption-stimulating factor produced by mouse fibrosarcoma cells is prostaglandin E 2 . A new model for the hypercalcemia of cancer. J Exp Med. 1972 Dec 1;136(6):1329–1343. doi: 10.1084/jem.136.6.1329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams N., Eger R. R., Moore M. A., Mendelsohn N. Differentiation of mouse bone marrow precursor cells into neutrophil granulocytes by an activity separation from WEHI-3 cell-conditioned medium. Differentiation. 1978 Jul 24;11(1):59–63. doi: 10.1111/j.1432-0436.1978.tb00970.x. [DOI] [PubMed] [Google Scholar]


