Abstract
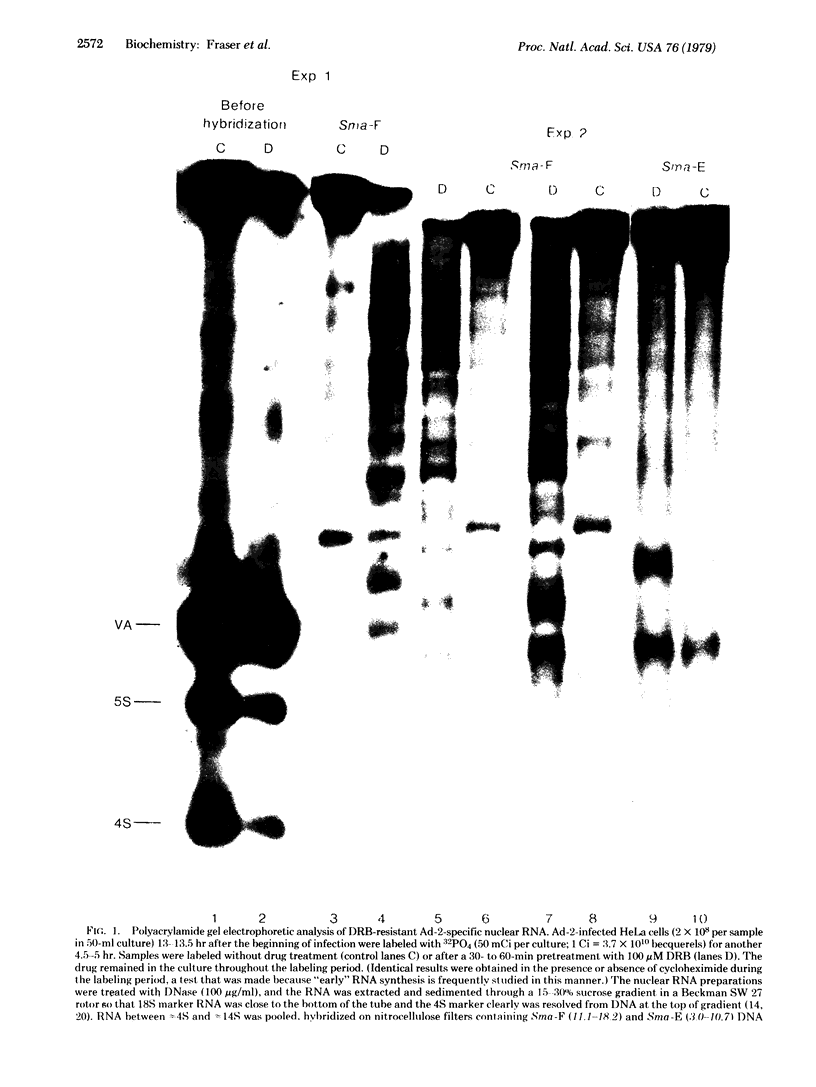
Discrete-sized short RNA chains that contain the distinctive oligonucleotides, including the 5'-capped oligonucleotide, characteristic of the first 600 nucleotides of the adenovirus type 2 (Ad-2) large, late, rightward-reading transcriptional unit (16.4-99) accumulate in Ad-2-infected HeLa cells. In the presence of 5,6-dichloro-1-beta-D-ribofuranosylbenzimidazole the accumulation of these chains is enhanced, as is the accumulation of short chains from a neighbouring rightward-reading transcriptional unit (3.0-10.7). These short chains appear to represent prematurely terminated transcripts. Late in infection there is a marked increase in RNA synthesis, including that of prematurely terminated short chains, from the large late transcriptional unit. This suggests that the increase in transcription and mRNA production from this region late in infection is not due to reduced "attenuation" of RNA synthesis.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Berget S. M., Moore C., Sharp P. A. Spliced segments at the 5' terminus of adenovirus 2 late mRNA. Proc Natl Acad Sci U S A. 1977 Aug;74(8):3171–3175. doi: 10.1073/pnas.74.8.3171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertrand K., Korn L., Lee F., Platt T., Squires C. L., Squires C., Yanofsky C. New features of the regulation of the tryptophan operon. Science. 1975 Jul 4;189(4196):22–26. doi: 10.1126/science.1094538. [DOI] [PubMed] [Google Scholar]
- Celma M. L., Pan J., Weissman S. M. Studies of low molecular weight RNA from cells infected with adenovirus 2. I. The sequences at the 3' end of VA-RNA I. J Biol Chem. 1977 Dec 25;252(24):9032–9042. [PubMed] [Google Scholar]
- Celma M. L., Pan J., Weissman S. M. Studies of low molecular weight RNA from cells infected with adenovirus 2. II. Heterogeneity at the 5' end of VA-RNA I. J Biol Chem. 1977 Dec 25;252(24):9043–9046. [PubMed] [Google Scholar]
- Chow L. T., Gelinas R. E., Broker T. R., Roberts R. J. An amazing sequence arrangement at the 5' ends of adenovirus 2 messenger RNA. Cell. 1977 Sep;12(1):1–8. doi: 10.1016/0092-8674(77)90180-5. [DOI] [PubMed] [Google Scholar]
- Chow L. T., Roberts J. M., Lewis J. B., Broker T. R. A map of cytoplasmic RNA transcripts from lytic adenovirus type 2, determined by electron microscopy of RNA:DNA hybrids. Cell. 1977 Aug;11(4):819–836. doi: 10.1016/0092-8674(77)90294-x. [DOI] [PubMed] [Google Scholar]
- Egyházi E. Inhibition of Balbiani ring RNA synthesis at the initiation level. Proc Natl Acad Sci U S A. 1975 Mar;72(3):947–950. doi: 10.1073/pnas.72.3.947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans R., Weber J., Ziff E., Darnell J. E. Premature termination during adenovirus transcription. Nature. 1979 Mar 22;278(5702):367–370. doi: 10.1038/278367a0. [DOI] [PubMed] [Google Scholar]
- Flint J. The topography and transcription of the adenovirus genome. Cell. 1977 Feb;10(2):153–166. doi: 10.1016/0092-8674(77)90211-2. [DOI] [PubMed] [Google Scholar]
- Forget B. G., Weissman S. M. Nucleotide sequence of KB cell 5S RNA. Science. 1967 Dec 29;158(3809):1695–1699. doi: 10.1126/science.158.3809.1695. [DOI] [PubMed] [Google Scholar]
- Fraser N. W., Sehgal P. B., Darnell J. E. DRB-induced premature termination of late adenovirus transcription. Nature. 1978 Apr 13;272(5654):590–593. doi: 10.1038/272590a0. [DOI] [PubMed] [Google Scholar]
- Fraser N., Ziff E. RNA structures near poly(A) of adenovirus-2 late messenger RNAs. J Mol Biol. 1978 Sep 5;124(1):27–31. doi: 10.1016/0022-2836(78)90145-6. [DOI] [PubMed] [Google Scholar]
- Gelinas R. E., Roberts R. J. One predominant 5'-undecanucleotide in adenovirus 2 late messenger RNAs. Cell. 1977 Jul;11(3):533–544. doi: 10.1016/0092-8674(77)90071-x. [DOI] [PubMed] [Google Scholar]
- Goldberg S., Weber J., Darnell J. E., Jr The definition of a large viral transcription unit late in Ad2 infection of HeLa cells: mapping by effects of ultraviolet irradiation. Cell. 1977 Apr;10(4):617–621. doi: 10.1016/0092-8674(77)90094-0. [DOI] [PubMed] [Google Scholar]
- Klessig D. F. Two adenovirus mRNAs have a common 5' terminal leader sequence encoded at least 10 kb upstream from their main coding regions. Cell. 1977 Sep;12(1):9–21. doi: 10.1016/0092-8674(77)90181-7. [DOI] [PubMed] [Google Scholar]
- Lewis J. B., Anderson C. W., Atkins J. F. Further mapping of late adenovirus genes by cell-free translation of RNA selected by hybridization to specific DNA fragments. Cell. 1977 Sep;12(1):37–44. doi: 10.1016/0092-8674(77)90183-0. [DOI] [PubMed] [Google Scholar]
- Loening U. E. The fractionation of high-molecular-weight ribonucleic acid by polyacrylamide-gel electrophoresis. Biochem J. 1967 Jan;102(1):251–257. doi: 10.1042/bj1020251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mills D. R., Dobkin C., Kramer F. R. Template-determined, variable rate of RNA chain elongation. Cell. 1978 Oct;15(2):541–550. doi: 10.1016/0092-8674(78)90022-3. [DOI] [PubMed] [Google Scholar]
- Nevins J. R., Darnell J. E. Groups of adenovirus type 2 mRNA's derived from a large primary transcript: probable nuclear origin and possible common 3' ends. J Virol. 1978 Mar;25(3):811–823. doi: 10.1128/jvi.25.3.811-823.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nevins J. R., Darnell J. E., Jr Steps in the processing of Ad2 mRNA: poly(A)+ nuclear sequences are conserved and poly(A) addition precedes splicing. Cell. 1978 Dec;15(4):1477–1493. doi: 10.1016/0092-8674(78)90071-5. [DOI] [PubMed] [Google Scholar]
- Philipson L., Pettersson U., Lindberg U., Tibbetts C., Vennström B., Persson T. RNA synthesis and processing in adenovirus-infected cells. Cold Spring Harb Symp Quant Biol. 1975;39(Pt 1):447–456. doi: 10.1101/sqb.1974.039.01.057. [DOI] [PubMed] [Google Scholar]
- Price R., Penman S. Transcription of the adenovirus genome by an -amanitine-sensitive ribonucleic acid polymerase in HeLa cells. J Virol. 1972 Apr;9(4):621–626. doi: 10.1128/jvi.9.4.621-626.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sehgal P. B., Darnell J. E., Jr, Tamm I. The inhibition by DRB (5,6-dichloro-1-beta-D-ribofuranosylbenzimidazole) of hnRNA and mRNA production in HeLa cells. Cell. 1976 Nov;9(3):473–480. doi: 10.1016/0092-8674(76)90092-1. [DOI] [PubMed] [Google Scholar]
- Sehgal P. B., Derman E., Molloy G. R., Tamm I., Darnell J. E. 5,6-Dichloro-1-Beta-D-ribofuranosylbenzimidazole inhibits initiation of nuclear heterogeneous RNA chains in HeLa cells. Science. 1976 Oct 22;194(4263):431–433. doi: 10.1126/science.982026. [DOI] [PubMed] [Google Scholar]
- Sehgal P. B., Fraser N. W., Darnell J. E., Jr Early Ad-2 transcription units: only promoter-proximal RNA continues to be made in the presence of DRB. Virology. 1979 Apr 15;94(1):185–191. doi: 10.1016/0042-6822(79)90448-3. [DOI] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Stauffer G. V., Zurawski G., Yanofsky C. Single base-pair alterations in the Escherichia coli trp operon leader region that relieve transcription termination at the trp attenuator. Proc Natl Acad Sci U S A. 1978 Oct;75(10):4833–4837. doi: 10.1073/pnas.75.10.4833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamm I. Definition of subclasses of nucleoplasmic RNA. Proc Natl Acad Sci U S A. 1977 Nov;74(11):5011–5015. doi: 10.1073/pnas.74.11.5011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wall R., Philipson L., Darnell J. E. Processing of adenovirus specific nuclear RNA during virus replication. Virology. 1972 Oct;50(1):27–34. doi: 10.1016/0042-6822(72)90342-x. [DOI] [PubMed] [Google Scholar]
- Wallace R. D., Kates J. State of adenovirus 2 deoxyribonucleic acid in the nucleus and its mode of transcription: studies with isolated viral deoxyribonucleic acid-protein complexes and isolated nuclei. J Virol. 1972 Apr;9(4):627–635. doi: 10.1128/jvi.9.4.627-635.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber J., Jelinek W., Darnell J. E., Jr The definition of a large viral transcription unit late in Ad2 infection of HeLa cells: mapping of nascent RNA molecules labeled in isolated nuclei. Cell. 1977 Apr;10(4):611–616. doi: 10.1016/0092-8674(77)90093-9. [DOI] [PubMed] [Google Scholar]
- Ziff E. B., Evans R. M. Coincidence of the promoter and capped 5' terminus of RNA from the adenovirus 2 major late transcription unit. Cell. 1978 Dec;15(4):1463–1475. doi: 10.1016/0092-8674(78)90070-3. [DOI] [PubMed] [Google Scholar]