Abstract
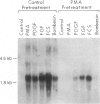
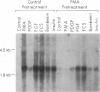
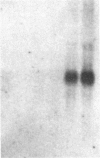
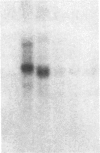
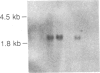
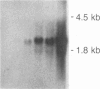
We investigated the expression of the protooncogene c-fos in 3T3-L1 fibroblasts and adipocytes in response to a variety of growth-promoting agents in normal cells and in cells preincubated with phorbol esters to deplete them of protein kinase C. There was a rapid accumulation of c-fos mRNA in fibroblasts and adipocytes treated with phorbol 12-myristate 13-acetate, platelet-derived growth factor, fibroblast growth factor, fetal calf serum, bombesin, and insulin, especially in the adipocytes. Phorbol 12-myristate 13-acetate pretreatment abolished the increase in c-fos mRNA due to additional phorbol 12-myristate 13-acetate treatment and decreased but did not eliminate the ability of platelet-derived growth factor, fibroblast growth factor, fetal calf serum, bombesin, and insulin to stimulate c-fos mRNA. These data suggested that c-fos mRNA could be induced in serum-deprived 3T3-L1 fibroblasts and adipocytes by at least two separate pathways, one involving protein kinase C and the other independent of protein kinase C. In the very insulin-sensitive 3T3-L1 adipocytes, insulin rapidly and transiently increased c-fos expression (c-fos mRNA appeared by 15 min and disappeared after 60 min) via interaction with its own cellular receptor, rather than by interacting with receptors for one of the insulin-like growth factors. Cycloheximide treatment in combination with insulin or phorbol 12-myristate 13-acetate resulted in superinduction of c-fos mRNA. We conclude that insulin can rapidly stimulate c-fos mRNA accumulation in 3T3-L1 adipocytes and that part of the growth factor-stimulated increase in this mRNA that occurs in protein kinase C-deficient cells may be due to activation of a pathway similar or identical to that activated by insulin.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alexander M., Curtis G., Avruch J., Goodman H. M. Insulin regulation of protein biosynthesis in differentiated 3T3 adipocytes. Regulation of glyceraldehyde-3-phosphate dehydrogenase. J Biol Chem. 1985 Oct 5;260(22):11978–11985. [PubMed] [Google Scholar]
- Andreone T. L., Beale E. G., Bar R. S., Granner D. K. Insulin decreases phosphoenolpyruvate carboxykinase (GTP) mRNA activity by a receptor-mediated process. J Biol Chem. 1982 Jan 10;257(1):35–38. [PubMed] [Google Scholar]
- Berridge M. J., Heslop J. P., Irvine R. F., Brown K. D. Inositol lipids and cell proliferation. Biochem Soc Trans. 1985 Feb;13(1):67–71. doi: 10.1042/bst0130067. [DOI] [PubMed] [Google Scholar]
- Besterman J. M., Watson S. P., Cuatrecasas P. Lack of association of epidermal growth factor-, insulin-, and serum-induced mitogenesis with stimulation of phosphoinositide degradation in BALB/c 3T3 fibroblasts. J Biol Chem. 1986 Jan 15;261(2):723–727. [PubMed] [Google Scholar]
- Blackshear P. J., Wen L., Glynn B. P., Witters L. A. Protein kinase C-stimulated phosphorylation in vitro of a Mr 80,000 protein phosphorylated in response to phorbol esters and growth factors in intact fibroblasts. Distinction from protein kinase C and prominence in brain. J Biol Chem. 1986 Jan 25;261(3):1459–1469. [PubMed] [Google Scholar]
- Blackshear P. J., Wen L., Nemenoff R. A., Gunsalus J. R., Witters L. A. Growth factor activation of protein kinase C-dependent and -independent pathways of protein phosphorylation in fibroblasts: relevance to activation of protein kinase C in neuronal tissues. Prog Brain Res. 1986;69:183–195. doi: 10.1016/s0079-6123(08)61059-6. [DOI] [PubMed] [Google Scholar]
- Blackshear P. J., Witters L. A., Girard P. R., Kuo J. F., Quamo S. N. Growth factor-stimulated protein phosphorylation in 3T3-L1 cells. Evidence for protein kinase C-dependent and -independent pathways. J Biol Chem. 1985 Oct 25;260(24):13304–13315. [PubMed] [Google Scholar]
- Blenis J., Erikson R. L. Stimulation of ribosomal protein S6 kinase activity by pp60v-src or by serum: dissociation from phorbol ester-stimulated activity. Proc Natl Acad Sci U S A. 1986 Mar;83(6):1733–1737. doi: 10.1073/pnas.83.6.1733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bravo R., Burckhardt J., Curran T., Müller R. Expression of c-fos in NIH3T3 cells is very low but inducible throughout the cell cycle. EMBO J. 1986 Apr;5(4):695–700. doi: 10.1002/j.1460-2075.1986.tb04269.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cochet C., Gill G. N., Meisenhelder J., Cooper J. A., Hunter T. C-kinase phosphorylates the epidermal growth factor receptor and reduces its epidermal growth factor-stimulated tyrosine protein kinase activity. J Biol Chem. 1984 Feb 25;259(4):2553–2558. [PubMed] [Google Scholar]
- Cochran B. H., Zullo J., Verma I. M., Stiles C. D. Expression of the c-fos gene and of an fos-related gene is stimulated by platelet-derived growth factor. Science. 1984 Nov 30;226(4678):1080–1082. doi: 10.1126/science.6093261. [DOI] [PubMed] [Google Scholar]
- Collins M. K., Rozengurt E. Homologous and heterologous mitogenic desensitization of Swiss 3T3 cells to phorbol esters and vasopressin: role of receptor and postreceptor steps. J Cell Physiol. 1984 Feb;118(2):133–142. doi: 10.1002/jcp.1041180205. [DOI] [PubMed] [Google Scholar]
- Coughlin S. R., Lee W. M., Williams P. W., Giels G. M., Williams L. T. c-myc gene expression is stimulated by agents that activate protein kinase C and does not account for the mitogenic effect of PDGF. Cell. 1985 Nov;43(1):243–251. doi: 10.1016/0092-8674(85)90029-7. [DOI] [PubMed] [Google Scholar]
- Curran T., Morgan J. I. Superinduction of c-fos by nerve growth factor in the presence of peripherally active benzodiazepines. Science. 1985 Sep 20;229(4719):1265–1268. doi: 10.1126/science.4035354. [DOI] [PubMed] [Google Scholar]
- Drake R. L., Mucenski C. G. Insulin mediates the asynchronous accumulation of hepatic albumin and malic enzyme messenger RNAs. Biochem Biophys Res Commun. 1985 Jul 16;130(1):317–324. doi: 10.1016/0006-291x(85)90420-6. [DOI] [PubMed] [Google Scholar]
- Flaim K. E., Hutson S. M., Lloyd C. E., Taylor J. M., Shiman R., Jefferson L. S. Direct effect of insulin on albumin gene expression in primary cultures of rat hepatocytes. Am J Physiol. 1985 Nov;249(5 Pt 1):E447–E453. doi: 10.1152/ajpendo.1985.249.5.E447. [DOI] [PubMed] [Google Scholar]
- Gonda T. J., Metcalf D. Expression of myb, myc and fos proto-oncogenes during the differentiation of a murine myeloid leukaemia. Nature. 1984 Jul 19;310(5974):249–251. doi: 10.1038/310249a0. [DOI] [PubMed] [Google Scholar]
- Granner D., Andreone T., Sasaki K., Beale E. Inhibition of transcription of the phosphoenolpyruvate carboxykinase gene by insulin. Nature. 1983 Oct 6;305(5934):549–551. doi: 10.1038/305549a0. [DOI] [PubMed] [Google Scholar]
- Greenberg M. E., Greene L. A., Ziff E. B. Nerve growth factor and epidermal growth factor induce rapid transient changes in proto-oncogene transcription in PC12 cells. J Biol Chem. 1985 Nov 15;260(26):14101–14110. [PubMed] [Google Scholar]
- Greenberg M. E., Hermanowski A. L., Ziff E. B. Effect of protein synthesis inhibitors on growth factor activation of c-fos, c-myc, and actin gene transcription. Mol Cell Biol. 1986 Apr;6(4):1050–1057. doi: 10.1128/mcb.6.4.1050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenberg M. E., Ziff E. B. Stimulation of 3T3 cells induces transcription of the c-fos proto-oncogene. Nature. 1984 Oct 4;311(5985):433–438. doi: 10.1038/311433a0. [DOI] [PubMed] [Google Scholar]
- Hovis J. G., Stumpo D. J., Halsey D. L., Blackshear P. J. Effects of mitogens on ornithine decarboxylase activity and messenger RNA levels in normal and protein kinase C-deficient NIH-3T3 fibroblasts. J Biol Chem. 1986 Aug 5;261(22):10380–10386. [PubMed] [Google Scholar]
- Iwashita S., Fox C. F. Epidermal growth factor and potent phorbol tumor promoters induce epidermal growth factor receptor phosphorylation in a similar but distinctively different manner in human epidermoid carcinoma A431 cells. J Biol Chem. 1984 Feb 25;259(4):2559–2567. [PubMed] [Google Scholar]
- Jacobs S., Sahyoun N. E., Saltiel A. R., Cuatrecasas P. Phorbol esters stimulate the phosphorylation of receptors for insulin and somatomedin C. Proc Natl Acad Sci U S A. 1983 Oct;80(20):6211–6213. doi: 10.1073/pnas.80.20.6211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaibuchi K., Tsuda T., Kikuchi A., Tanimoto T., Yamashita T., Takai Y. Possible involvement of protein kinase C and calcium ion in growth factor-induced expression of c-myc oncogene in Swiss 3T3 fibroblasts. J Biol Chem. 1986 Jan 25;261(3):1187–1192. [PubMed] [Google Scholar]
- Karlsson F. A., Grunfeld C., Kahn C. R., Roth J. Regulation of insulin receptors and insulin responsiveness in 3T3-L1 fatty fibroblasts. Endocrinology. 1979 May;104(5):1383–1392. doi: 10.1210/endo-104-5-1383. [DOI] [PubMed] [Google Scholar]
- Kelly K., Cochran B. H., Stiles C. D., Leder P. Cell-specific regulation of the c-myc gene by lymphocyte mitogens and platelet-derived growth factor. Cell. 1983 Dec;35(3 Pt 2):603–610. doi: 10.1016/0092-8674(83)90092-2. [DOI] [PubMed] [Google Scholar]
- Korc M., Owerbach D., Quinto C., Rutter W. J. Pancreatic islet-acinar cell interaction: amylase messenger RNA levels ar determined by insulin. Science. 1981 Jul 17;213(4505):351–353. doi: 10.1126/science.6166044. [DOI] [PubMed] [Google Scholar]
- Kruijer W., Cooper J. A., Hunter T., Verma I. M. Platelet-derived growth factor induces rapid but transient expression of the c-fos gene and protein. Nature. 1984 Dec 20;312(5996):711–716. doi: 10.1038/312711a0. [DOI] [PubMed] [Google Scholar]
- Kruijer W., Schubert D., Verma I. M. Induction of the proto-oncogene fos by nerve growth factor. Proc Natl Acad Sci U S A. 1985 Nov;82(21):7330–7334. doi: 10.1073/pnas.82.21.7330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kruijer W., Skelly H., Botteri F., van der Putten H., Barber J. R., Verma I. M., Leffert H. L. Proto-oncogene expression in regenerating liver is simulated in cultures of primary adult rat hepatocytes. J Biol Chem. 1986 Jun 15;261(17):7929–7933. [PubMed] [Google Scholar]
- L'Allemain G., Pouysségur EGF and insulin action in fibroblasts. Evidence that phosphoinositide hydrolysis is not an essential mitogenic signalling pathway. FEBS Lett. 1986 Mar 3;197(1-2):344–348. doi: 10.1016/0014-5793(86)80354-4. [DOI] [PubMed] [Google Scholar]
- Marquardt H., Todaro G. J., Henderson L. E., Oroszlan S. Purification and primary structure of a polypeptide with multiplication-stimulating activity from rat liver cell cultures. Homology with human insulin-like growth factor II. J Biol Chem. 1981 Jul 10;256(13):6859–6865. [PubMed] [Google Scholar]
- Massagué J., Czech M. P. The subunit structures of two distinct receptors for insulin-like growth factors I and II and their relationship to the insulin receptor. J Biol Chem. 1982 May 10;257(9):5038–5045. [PubMed] [Google Scholar]
- Messina J. L., Hamlin J., Azizkahn J., Larner J. The effects of insulin and concanavalin A on the accumulation of a specific mRNA in rat hepatoma cells. Biochem Biophys Res Commun. 1985 Dec 31;133(3):1168–1174. doi: 10.1016/0006-291x(85)91259-8. [DOI] [PubMed] [Google Scholar]
- Messina J. L., Hamlin J., Larner J. Effects of insulin alone on the accumulation of a specific mRNA in rat hepatoma cells. J Biol Chem. 1985 Dec 25;260(30):16418–16423. [PubMed] [Google Scholar]
- Milbrandt J. Nerve growth factor rapidly induces c-fos mRNA in PC12 rat pheochromocytoma cells. Proc Natl Acad Sci U S A. 1986 Jul;83(13):4789–4793. doi: 10.1073/pnas.83.13.4789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell R. L., Henning-Chubb C., Huberman E., Verma I. M. c-fos expression is neither sufficient nor obligatory for differentiation of monomyelocytes to macrophages. Cell. 1986 May 23;45(4):497–504. doi: 10.1016/0092-8674(86)90281-3. [DOI] [PubMed] [Google Scholar]
- Mitchell R. L., Zokas L., Schreiber R. D., Verma I. M. Rapid induction of the expression of proto-oncogene fos during human monocytic differentiation. Cell. 1985 Jan;40(1):209–217. doi: 10.1016/0092-8674(85)90324-1. [DOI] [PubMed] [Google Scholar]
- Müller R., Bravo R., Burckhardt J., Curran T. Induction of c-fos gene and protein by growth factors precedes activation of c-myc. Nature. 1984 Dec 20;312(5996):716–720. doi: 10.1038/312716a0. [DOI] [PubMed] [Google Scholar]
- Müller R. Cellular and viral fos genes: structure, regulation of expression and biological properties of their encoded products. Biochim Biophys Acta. 1986;823(3):207–225. doi: 10.1016/0304-419x(86)90003-x. [DOI] [PubMed] [Google Scholar]
- Müller R., Curran T., Müller D., Guilbert L. Induction of c-fos during myelomonocytic differentiation and macrophage proliferation. Nature. 1985 Apr 11;314(6011):546–548. doi: 10.1038/314546a0. [DOI] [PubMed] [Google Scholar]
- Müller R., Wagner E. F. Differentiation of F9 teratocarcinoma stem cells after transfer of c-fos proto-oncogenes. Nature. 1984 Oct 4;311(5985):438–442. doi: 10.1038/311438a0. [DOI] [PubMed] [Google Scholar]
- Noguchi T., Inoue H., Chen H. L., Matsubara K., Tanaka T. Molecular cloning of DNA complementary to rat L-type pyruvate kinase mRNA. Nutritional and hormonal regulation of L-type pyruvate kinase mRNA concentration. J Biol Chem. 1983 Dec 25;258(24):15220–15223. [PubMed] [Google Scholar]
- Pelech S. L., Olwin B. B., Krebs E. G. Fibroblast growth factor treatment of Swiss 3T3 cells activates a subunit S6 kinase that phosphorylates a synthetic peptide substrate. Proc Natl Acad Sci U S A. 1986 Aug;83(16):5968–5972. doi: 10.1073/pnas.83.16.5968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reed B. C., Kaufmann S. H., Mackall J. C., Student A. K., Lane M. D. Alterations in insulin binding accompanying differentiation of 3T3-L1 preadipocytes. Proc Natl Acad Sci U S A. 1977 Nov;74(11):4876–4880. doi: 10.1073/pnas.74.11.4876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rozengurt E., Rodriguez-Pena A., Coombs M., Sinnett-Smith J. Diacylglycerol stimulates DNA synthesis and cell division in mouse 3T3 cells: role of Ca2+-sensitive phospholipid-dependent protein kinase. Proc Natl Acad Sci U S A. 1984 Sep;81(18):5748–5752. doi: 10.1073/pnas.81.18.5748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rozengurt E., Rodriguez-Pena M., Smith K. A. Phorbol esters, phospholipase C, and growth factors rapidly stimulate the phosphorylation of a Mr 80,000 protein in intact quiescent 3T3 cells. Proc Natl Acad Sci U S A. 1983 Dec;80(23):7244–7248. doi: 10.1073/pnas.80.23.7244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubin C. S., Hirsch A., Fung C., Rosen O. M. Development of hormone receptors and hormonal responsiveness in vitro. Insulin receptors and insulin sensitivity in the preadipocyte and adipocyte forms of 3T3-L1 cells. J Biol Chem. 1978 Oct 25;253(20):7570–7578. [PubMed] [Google Scholar]
- Rubin C. S., Lai E., Rosen O. M. Acquisition of increased hormone sensitivity during in vitro adipocyte development. J Biol Chem. 1977 May 25;252(10):3554–3557. [PubMed] [Google Scholar]
- Rüther U., Wagner E. F., Müller R. Analysis of the differentiation-promoting potential of inducible c-fos genes introduced into embryonal carcinoma cells. EMBO J. 1985 Jul;4(7):1775–1781. doi: 10.1002/j.1460-2075.1985.tb03850.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasaki K., Cripe T. P., Koch S. R., Andreone T. L., Petersen D. D., Beale E. G., Granner D. K. Multihormonal regulation of phosphoenolpyruvate carboxykinase gene transcription. The dominant role of insulin. J Biol Chem. 1984 Dec 25;259(24):15242–15251. [PubMed] [Google Scholar]
- Setoyama C., Frunzio R., Liau G., Mudryj M., de Crombrugghe B. Transcriptional activation encoded by the v-fos gene. Proc Natl Acad Sci U S A. 1986 May;83(10):3213–3217. doi: 10.1073/pnas.83.10.3213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stumpo D. J., Kletzien R. F. Regulation of glucose-6-phosphate dehydrogenase mRNA by insulin and the glucocorticoids in primary cultures of rat hepatocytes. Eur J Biochem. 1984 Nov 2;144(3):497–502. doi: 10.1111/j.1432-1033.1984.tb08493.x. [DOI] [PubMed] [Google Scholar]
- Tabarini D., Heinrich J., Rosen O. M. Activation of S6 kinase activity in 3T3-L1 cells by insulin and phorbol ester. Proc Natl Acad Sci U S A. 1985 Jul;82(13):4369–4373. doi: 10.1073/pnas.82.13.4369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takayama S., White M. F., Lauris V., Kahn C. R. Phorbol esters modulate insulin receptor phosphorylation and insulin action in cultured hepatoma cells. Proc Natl Acad Sci U S A. 1984 Dec;81(24):7797–7801. doi: 10.1073/pnas.81.24.7797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treisman R. Transient accumulation of c-fos RNA following serum stimulation requires a conserved 5' element and c-fos 3' sequences. Cell. 1985 Oct;42(3):889–902. doi: 10.1016/0092-8674(85)90285-5. [DOI] [PubMed] [Google Scholar]
- Tsuda T., Kaibuchi K., Kawahara Y., Fukuzaki H., Takai Y. Induction of protein kinase C activation and Ca2+ mobilization by fibroblast growth factor in Swiss 3T3 cells. FEBS Lett. 1985 Oct 28;191(2):205–210. doi: 10.1016/0014-5793(85)80009-0. [DOI] [PubMed] [Google Scholar]
- Verma S. S., Gupta R. K., Kishore N., Sen Gupta J. A simple relationship between maximal aerobic power and body weight in Indian adolescent boys. Indian J Med Sci. 1986 Apr;40(4):93–96. [PubMed] [Google Scholar]
- Volpe J. J., Vagelos P. R. Mechanisms and regulation of biosynthesis of saturated fatty acids. Physiol Rev. 1976 Apr;56(2):339–417. doi: 10.1152/physrev.1976.56.2.339. [DOI] [PubMed] [Google Scholar]
- Yoo-Warren H., Cimbala M. A., Felz K., Monahan J. E., Leis J. P., Hanson R. W. Identification of a DNA clone to phosphoenolpyruvate carboxykinase (GTP) from rat cytosol. Alterations in phosphoenolpyruvate carboxykinase RNA levels detectable by hybridization. J Biol Chem. 1981 Oct 25;256(20):10224–10227. [PubMed] [Google Scholar]
- Zachary I., Sinnett-Smith J. W., Rozengurt E. Early events elicited by bombesin and structurally related peptides in quiescent Swiss 3T3 cells. I. Activation of protein kinase C and inhibition of epidermal growth factor binding. J Cell Biol. 1986 Jun;102(6):2211–2222. doi: 10.1083/jcb.102.6.2211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van de Werve G., Proietto J., Jeanrenaud B. Tumour-promoting phorbol esters increase basal and inhibit insulin-stimulated lipogenesis in rat adipocytes without decreasing insulin binding. Biochem J. 1985 Jan 15;225(2):523–527. doi: 10.1042/bj2250523. [DOI] [PMC free article] [PubMed] [Google Scholar]