Abstract
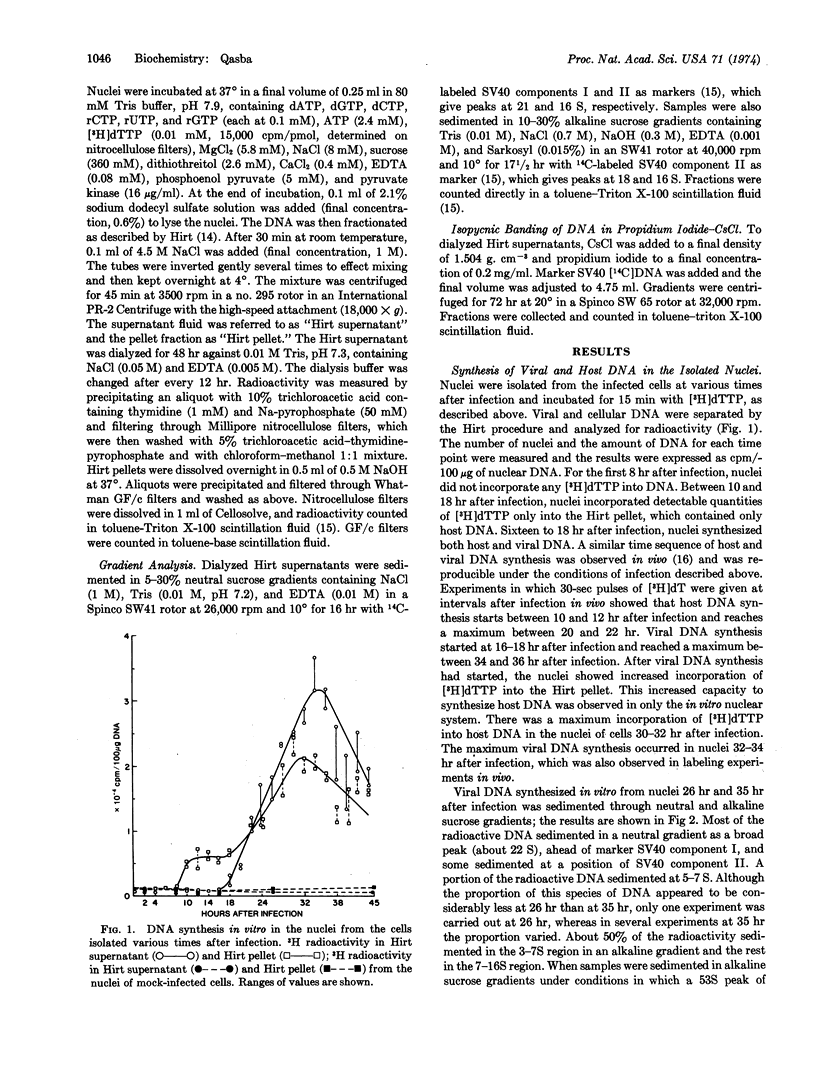
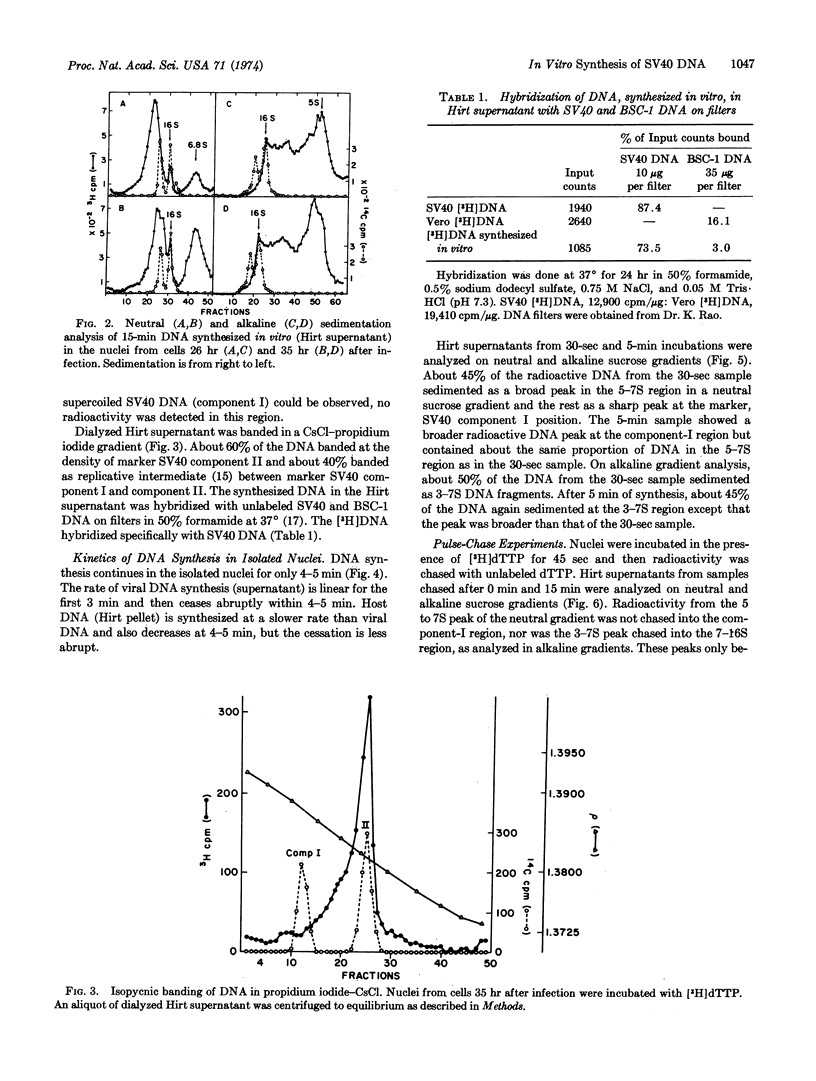
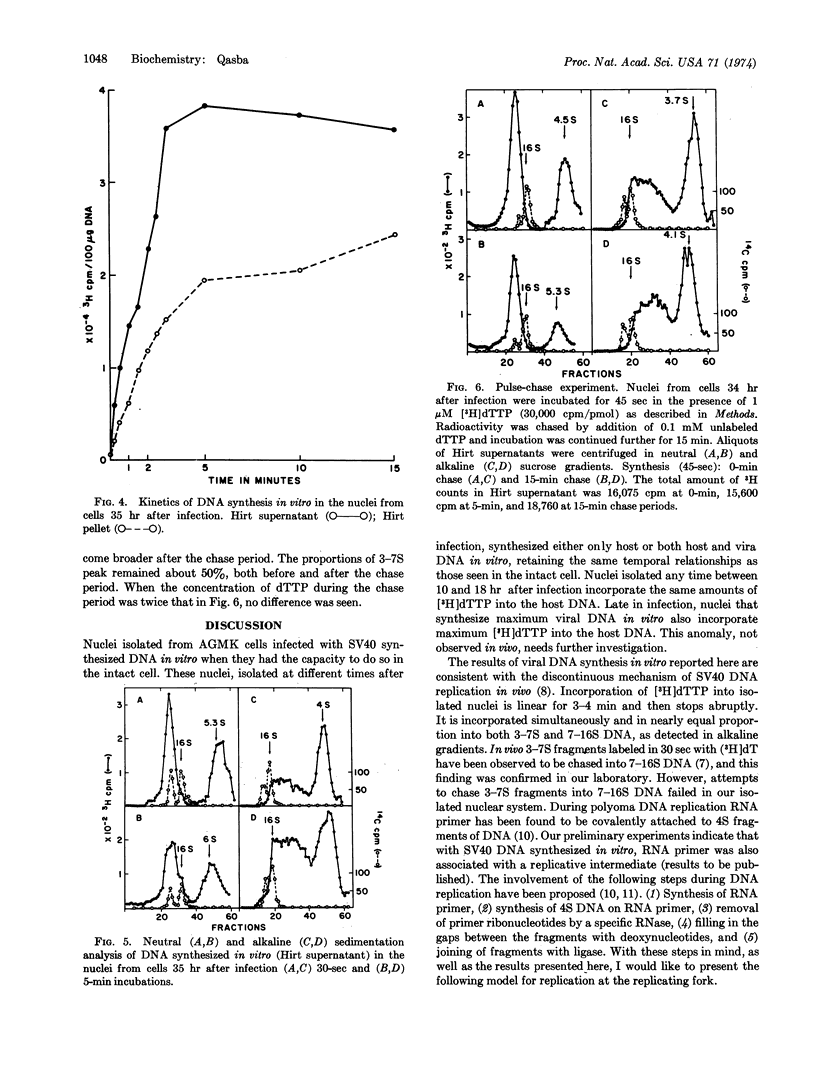
Nuclei isolated from African green monkey kidney cells infected with simian virus 40 at different times after infection maintain in vitro the same temporal sequence of host and viral DNA synthesis as that seen in intact cells. The viral DNA synthesized by the nuclei of cells previously infected for 32-35 hr was characterized by centrifugation through neutral and alkaline sucrose gradients, and by isopycnic banding in a propidium iodide-cesium chloride gradient. DNA synthesis in this system is maintained for only 4-5 min. Neutral sucrose gradient analysis showed that most of the radioactivity is associated with the replicative intermediate of simian virus 40 DNA and the rest sediments at 5-7 S. Alkaline gradient analysis showed that 50-60% of the radioactivity sediments as 3-7S fragments, and the rest between 7 and 16 S. Pulsechase experiments showed that in this system 3-7S fragments do not mature into long chains. A model is presented to explain the failure of these fragments to join into long chains in this in vitro nuclear system.
Keywords: discontinuous synthesis, 4S fragments, DNA maturation
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BURTON K. A study of the conditions and mechanism of the diphenylamine reaction for the colorimetric estimation of deoxyribonucleic acid. Biochem J. 1956 Feb;62(2):315–323. doi: 10.1042/bj0620315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danna K. J., Nathans D. Bidirectional replication of Simian Virus 40 DNA. Proc Natl Acad Sci U S A. 1972 Nov;69(11):3097–3100. doi: 10.1073/pnas.69.11.3097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fareed G. C., Khoury G., Salzman N. P. Self-annealing of 4 S strands from replicating simian virus 40 DNA. J Mol Biol. 1973 Jul 5;77(3):457–462. doi: 10.1016/0022-2836(73)90451-8. [DOI] [PubMed] [Google Scholar]
- Fareed G. C., Salzman N. P. Intermediate in SV40 DNA chain growth. Nat New Biol. 1972 Aug 30;238(87):274–277. doi: 10.1038/newbio238274a0. [DOI] [PubMed] [Google Scholar]
- Friedman D. L., Mueller G. C. A nuclear system for DNA replication from synchronized HeLa cells. Biochim Biophys Acta. 1968 Jul 23;161(2):455–468. doi: 10.1016/0005-2787(68)90122-6. [DOI] [PubMed] [Google Scholar]
- Hirt B. Selective extraction of polyoma DNA from infected mouse cell cultures. J Mol Biol. 1967 Jun 14;26(2):365–369. doi: 10.1016/0022-2836(67)90307-5. [DOI] [PubMed] [Google Scholar]
- Kidwell W. R. Fidelity of DNA replication in isolated L-cell nuclei. Biochim Biophys Acta. 1972 Apr 26;269(1):51–61. doi: 10.1016/0005-2787(72)90073-1. [DOI] [PubMed] [Google Scholar]
- Kidwell W. R., Mueller G. C. The synthesis and assembly of DNA subunits in isolated HeLa cell nuclei. Biochem Biophys Res Commun. 1969 Aug 22;36(5):756–763. doi: 10.1016/0006-291x(69)90674-3. [DOI] [PubMed] [Google Scholar]
- Lavi S., Winocour E. Acquisition of sequences homologous to host deoxyribonucleic acid by closed circular simian virus 40 deoxyribonucleic acid. J Virol. 1972 Feb;9(2):309–316. doi: 10.1128/jvi.9.2.309-316.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch W. E., Brown R. F., Umeda T., Langreth S. G., Lieberman I. Synthesis of deoxyribonucleic acid by isolated liver nuclei. J Biol Chem. 1970 Aug 10;245(15):3911–3916. [PubMed] [Google Scholar]
- Magnusson G. Hydroxyurea-induced accumulation of short fragments during polyoma DNA replication. I. Characterization of fragments. J Virol. 1973 Sep;12(3):600–608. doi: 10.1128/jvi.12.3.600-608.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magnusson G., Pigiet V., Winnacker E. L., Abrams R., Reichard P. RNA-linked short DNA fragments during polyoma replication. Proc Natl Acad Sci U S A. 1973 Feb;70(2):412–415. doi: 10.1073/pnas.70.2.412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magnusson G., Winnacker E. L., Eliasson R., Reichard P. Replication of polyoma DNA in isolated nuclei. II. Evidence for semi-conservative replication. J Mol Biol. 1972 Dec 30;72(3):539–552. doi: 10.1016/0022-2836(72)90173-8. [DOI] [PubMed] [Google Scholar]
- Ritzi E., Levine A. J. Deoxyribonucleic acid replication in simian virus 40-infected cells. 3. Comparison of simian virus 40 lytic infection in three different monkey kidney cell lines. J Virol. 1970 Jun;5(6):686–692. doi: 10.1128/jvi.5.6.686-692.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salzman N. P., Thoren M. M. Inhibition in the joining of DNA intermediates to growing simian virus 40 chains. J Virol. 1973 May;11(5):721–729. doi: 10.1128/jvi.11.5.721-729.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sebring E. D., Kelly T. J., Jr, Thoren M. M., Salzman N. P. Structure of replicating simian virus 40 deoxyribonucleic acid molecules. J Virol. 1971 Oct;8(4):478–490. doi: 10.1128/jvi.8.4.478-490.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winnacker E. L., Magnusson G., Reichard P. Replication of polyoma DNA in isolated nuclei. I. Characterization of the system from mouse fibroblast 3T6 cells. J Mol Biol. 1972 Dec 30;72(3):523–537. doi: 10.1016/0022-2836(72)90172-6. [DOI] [PubMed] [Google Scholar]


