Abstract
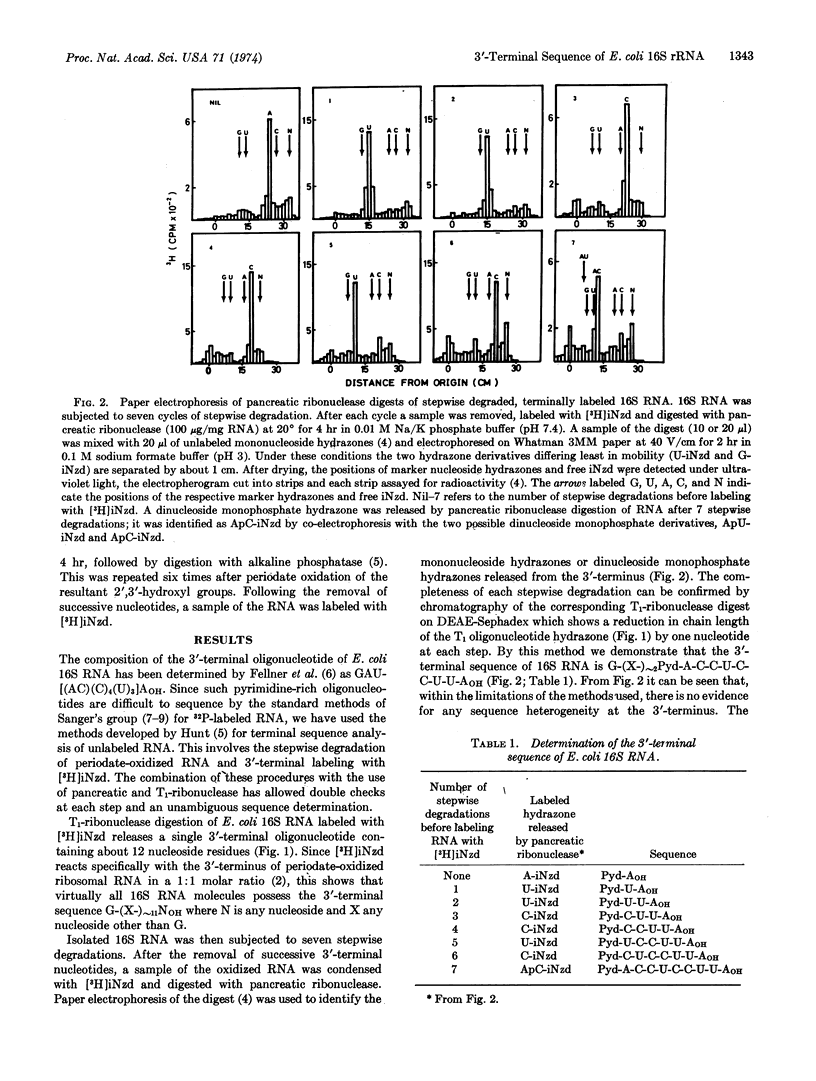
With a stepwise degradation and terminal labeling procedure the 3′-terminal sequence of E. coli 16S ribosomal RNA is shown to be Pyd-A-C-C-U-C-C-U-U-AOH. It is suggested that this region of the RNA is able to interact with mRNA and that the 3′-terminal U-U-AOH is involved in the termination of protein synthesis through base-pairing with terminator codons. The sequence A-C-C-U-C-C could recognize a conserved sequence found in the ribosome binding sites of various coliphage mRNAs; it may thus be involved in the formation of the mRNA·30S subunit complex.
Keywords: terminal labeling, stepwise degradation, protein synthesis, suppression
Full text
PDF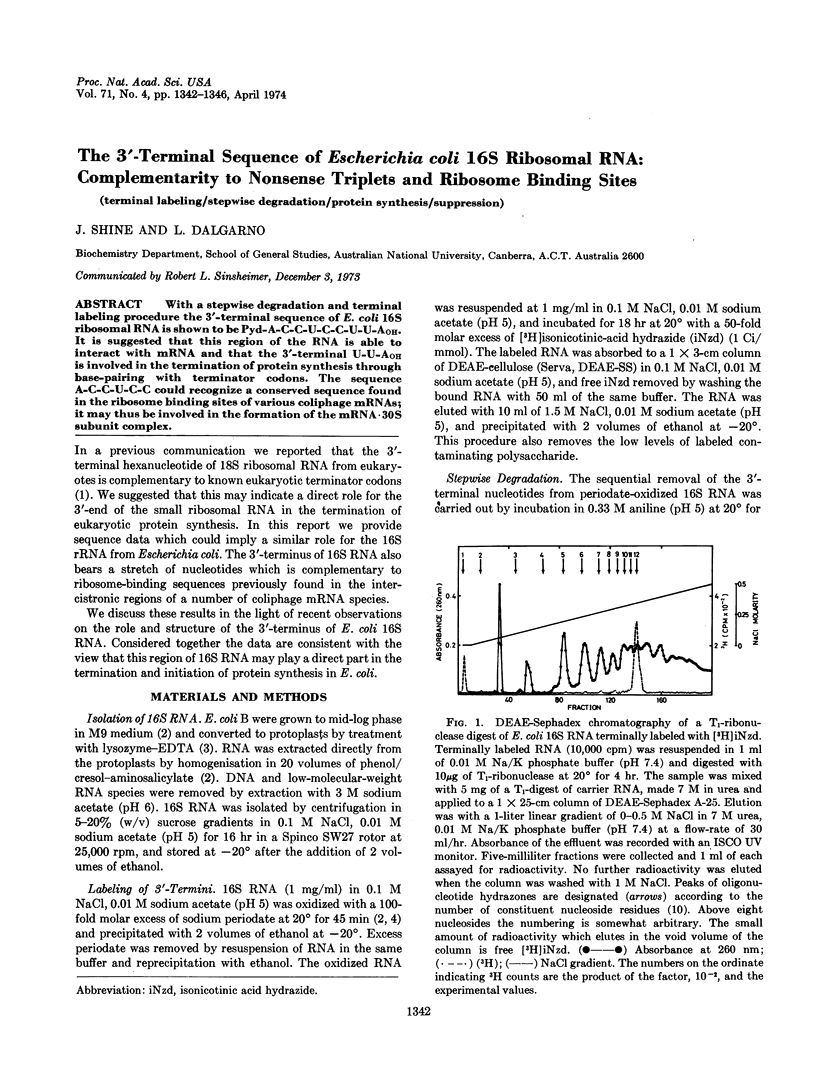



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arrand J. R., Hindley J. Nucleotide sequence of a ribosome binding site on RNA synthesized in vitro from coliphage T7. Nat New Biol. 1973 Jul 4;244(131):10–13. doi: 10.1038/newbio244010a0. [DOI] [PubMed] [Google Scholar]
- Boon T. Inactivation of ribosomes in vitro by colicin E 3 . Proc Natl Acad Sci U S A. 1971 Oct;68(10):2421–2425. doi: 10.1073/pnas.68.10.2421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boon T. Inactivation of ribosomes in vitro by colicin E 3 and its mechanism of action. Proc Natl Acad Sci U S A. 1972 Mar;69(3):549–552. doi: 10.1073/pnas.69.3.549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowman C. M., Dahlberg J. E., Ikemura T., Konisky J., Nomura M. Specific inactivation of 16S ribosomal RNA induced by colicin E3 in vivo. Proc Natl Acad Sci U S A. 1971 May;68(5):964–968. doi: 10.1073/pnas.68.5.964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenner S., Stretton A. O., Kaplan S. Genetic code: the 'nonsense' triplets for chain termination and their suppression. Nature. 1965 Jun 5;206(988):994–998. doi: 10.1038/206994a0. [DOI] [PubMed] [Google Scholar]
- Bronson M. J., Squires C., Yanofsky C. Nucleotide sequences from tryptophan messenger RNA of Escherichia coli: the sequence corresponding to the amino-terminal region of the first polypeptide specified by the operon. Proc Natl Acad Sci U S A. 1973 Aug;70(8):2335–2339. doi: 10.1073/pnas.70.8.2335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brownlee G. G., Sanger F., Barrell B. G. The sequence of 5 s ribosomal ribonucleic acid. J Mol Biol. 1968 Jun 28;34(3):379–412. doi: 10.1016/0022-2836(68)90168-x. [DOI] [PubMed] [Google Scholar]
- Capecchi M. R., Klein H. A. Characterization of three proteins involved in polypeptide chain termination. Cold Spring Harb Symp Quant Biol. 1969;34:469–477. doi: 10.1101/sqb.1969.034.01.053. [DOI] [PubMed] [Google Scholar]
- Chuang D., Simpson M. V. A translocation-associated ribosomal conformational change detected by hydrogen exchange and sedimentation velocity. Proc Natl Acad Sci U S A. 1971 Jul;68(7):1474–1478. doi: 10.1073/pnas.68.7.1474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Contreras R., Ysebaert M., Jou W. M., Fiers W. Bacteriophage Ms2 RNA: nucleotide sequence of the end of the a protein gene and the intercistronic region. Nat New Biol. 1973 Jan 24;241(108):99–101. doi: 10.1038/newbio241099a0. [DOI] [PubMed] [Google Scholar]
- Crick F. H. Codon--anticodon pairing: the wobble hypothesis. J Mol Biol. 1966 Aug;19(2):548–555. doi: 10.1016/s0022-2836(66)80022-0. [DOI] [PubMed] [Google Scholar]
- Dalgarno L., Shine J. Conserved terminal sequence in 18SrRNA may represent terminator anticodons. Nat New Biol. 1973 Oct 31;245(148):261–262. doi: 10.1038/newbio245261a0. [DOI] [PubMed] [Google Scholar]
- Fellner P., Ehresmann C., Stiegler P., Ebel J. P. Partial nucleotide sequence of 16S ribosomal RNA from E. coli. Nat New Biol. 1972 Sep 6;239(88):1–5. [PubMed] [Google Scholar]
- Ferretti J. J. Low-level reading of the UGA triplet in Salmonella typhimurium. J Bacteriol. 1971 May;106(2):691–693. doi: 10.1128/jb.106.2.691-693.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flessel C. P., Ralph P., Rich A. Polyribosomes of growing bacteria. Science. 1967 Nov 3;158(3801):658–660. doi: 10.1126/science.158.3801.658. [DOI] [PubMed] [Google Scholar]
- Gallucci E., Garen A. Suppressor genes for nonsense mutations. II. The su-4 and su-5 suppressor genes of Escherichia coli. J Mol Biol. 1966 Jan;15(1):193–200. doi: 10.1016/s0022-2836(66)80220-6. [DOI] [PubMed] [Google Scholar]
- Ganoza M. C., Tomkins J. K. Polypeptide chain termination in vitro: competition for nonsense codons between a purified release factor and suppressor tRNA. Biochem Biophys Res Commun. 1970 Sep 30;40(6):1455–1463. doi: 10.1016/0006-291x(70)90031-8. [DOI] [PubMed] [Google Scholar]
- Garen A., Garen S., Wilhelm R. C. Suppressor genes for nonsense mutations. I. The Su-1, Su-2 and Su-3 genes of Escherichia coli. J Mol Biol. 1965 Nov;14(1):167–178. doi: 10.1016/s0022-2836(65)80238-8. [DOI] [PubMed] [Google Scholar]
- Gupta S. L., Chen J., Schaefer L., Lengyel P., Weissman S. M. Nucleotide sequence of a ribosome attachment site of bacteriophage f2 RNA. Biochem Biophys Res Commun. 1970 Jun 5;39(5):883–888. doi: 10.1016/0006-291x(70)90406-7. [DOI] [PubMed] [Google Scholar]
- HUNT J. A. TERMINAL-SEQUENCE STUDIES OF HIGH-MOLECULAR-WEIGHT RIBONUCLEIC. THE REACTION OF PERIODATE-OXIDIZED RIBONUCLEOSIDES , 5'-RIBONUCLEOTIDES AND RIBONUCLEIC ACID WITH ISONIAZID. Biochem J. 1965 May;95:541–551. doi: 10.1042/bj0950541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helser T. L., Davies J. E., Dahlberg J. E. Change in methylation of 16S ribosomal RNA associated with mutation to kasugamycin resistance in Escherichia coli. Nat New Biol. 1971 Sep 1;233(35):12–14. doi: 10.1038/newbio233012a0. [DOI] [PubMed] [Google Scholar]
- Hirsh D., Gold L. Translation of the UGA triplet in vitro by tryptophan transfer RNA's. J Mol Biol. 1971 Jun 14;58(2):459–468. doi: 10.1016/0022-2836(71)90363-9. [DOI] [PubMed] [Google Scholar]
- Hunt J. A. Terminal sequence studies of high-molecular-weight ribonucleic acid. The 3' termini of rabbit globin messenger ribonucleic acid. Biochem J. 1973 Feb;131(2):315–325. doi: 10.1042/bj1310315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunt J. A. Terminal-sequence studies of high-molecular-weight ribonucleic acid. The 3'-termini of rabbit reticulocyte ribosomal RNA. Biochem J. 1970 Nov;120(2):353–363. doi: 10.1042/bj1200353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan S., Stretton A. O., Brenner S. Amber suppressors: efficiency of chain propagation and suppressor specific amino acids. J Mol Biol. 1965 Dec;14(2):528–533. doi: 10.1016/s0022-2836(65)80202-9. [DOI] [PubMed] [Google Scholar]
- Konisky J., Nomura M. Interaction of colicins with bacterial cells. II. Specific alteration of Escherichia coli ribosomes induced by colicin E3 in vivo. J Mol Biol. 1967 Jun 14;26(2):181–195. doi: 10.1016/0022-2836(67)90290-2. [DOI] [PubMed] [Google Scholar]
- LIPSETT M. N. COMPLEX FORMATION BETWEEN POLYCYTIDYLIC ACID AND GUANINE OLIGONUCLEOTIDES. J Biol Chem. 1964 Apr;239:1256–1260. [PubMed] [Google Scholar]
- Leffler S., Szer W. Messenger selection by bacterial ribosomes. Proc Natl Acad Sci U S A. 1973 Aug;70(8):2364–2368. doi: 10.1073/pnas.70.8.2364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lodish H. F. Bacteriophage f2 RNA: control of translation and gene order. Nature. 1968 Oct 26;220(5165):345–350. doi: 10.1038/220345a0. [DOI] [PubMed] [Google Scholar]
- Lodish H. F. Specificity in bacterial protein synthesis: role of initiation factors and ribosomal subunits. Nature. 1970 May 23;226(5247):705–707. doi: 10.1038/226705a0. [DOI] [PubMed] [Google Scholar]
- Min Jou W., Haegeman G., Ysebaert M., Fiers W. Nucleotide sequence of the gene coding for the bacteriophage MS2 coat protein. Nature. 1972 May 12;237(5350):82–88. doi: 10.1038/237082a0. [DOI] [PubMed] [Google Scholar]
- Model P., Webster R. E., Zinder N. D. The UGA codon in vitro: chain termination and suppression. J Mol Biol. 1969 Jul 14;43(1):177–190. doi: 10.1016/0022-2836(69)90087-4. [DOI] [PubMed] [Google Scholar]
- Moore C. H., Farron F., Bohnert D., Weissmann C. Possible origin of a minor virus specific protein (A1) in Q-beta particles. Nat New Biol. 1971 Sep 15;234(50):204–206. doi: 10.1038/newbio234204a0. [DOI] [PubMed] [Google Scholar]
- Okuyama A., Machiyama N., Kinoshita T., Tanaka N. Inhibition by kasugamycin of initiation complex formation on 30S ribosomes. Biochem Biophys Res Commun. 1971 Apr 2;43(1):196–199. doi: 10.1016/s0006-291x(71)80106-7. [DOI] [PubMed] [Google Scholar]
- Roth J. R. UGA nonsense mutations in Salmonella typhimurium. J Bacteriol. 1970 May;102(2):467–475. doi: 10.1128/jb.102.2.467-475.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sambrook J. F., Fan D. P., Brenner S. A strong suppressor specific for UGA. Nature. 1967 Apr 29;214(5087):452–453. doi: 10.1038/214452a0. [DOI] [PubMed] [Google Scholar]
- Sanger F., Brownlee G. G., Barrell B. G. A two-dimensional fractionation procedure for radioactive nucleotides. J Mol Biol. 1965 Sep;13(2):373–398. doi: 10.1016/s0022-2836(65)80104-8. [DOI] [PubMed] [Google Scholar]
- Santer U. V., Santer M. The sequence of the 3'-OH end of the 16 S RNA of Escherichia coli. FEBS Lett. 1972 Apr 1;21(3):311–314. doi: 10.1016/0014-5793(72)80191-1. [DOI] [PubMed] [Google Scholar]
- Schreier M. H., Noll H. Conformational changes in ribosomes during protein synthesis. Proc Natl Acad Sci U S A. 1971 Apr;68(4):805–809. doi: 10.1073/pnas.68.4.805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Senior B. W., Holland I. B. Effect of colicin E3 upon the 30S ribosomal subunit of Escherichia coli. Proc Natl Acad Sci U S A. 1971 May;68(5):959–963. doi: 10.1073/pnas.68.5.959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Senior B. W., Kwasniak J., Holland I. B. Colicin E3-directed changes in ribosome function and polyribosome metabolism in Escherichia coli K12. J Mol Biol. 1970 Oct 28;53(2):205–220. doi: 10.1016/0022-2836(70)90295-0. [DOI] [PubMed] [Google Scholar]
- Sherman M. I., Simpson M. V. Ribosomal conformation changes during subunit dissociation and reassociation. Cold Spring Harb Symp Quant Biol. 1969;34:220–222. [PubMed] [Google Scholar]
- Shine J., Dalgarno L. Occurrence of heat-dissociable ribosomal RNA in insects: the presence of three polynucleotide chains in 26 S RNA from cultured Aedes aegypti cells. J Mol Biol. 1973 Mar 25;75(1):57–72. doi: 10.1016/0022-2836(73)90528-7. [DOI] [PubMed] [Google Scholar]
- Sparling P. F. Kasugamycin resistance: 30S ribosomal mutation with an unusual location on the Escherichia coli chromosome. Science. 1970 Jan 2;167(3914):56–58. doi: 10.1126/science.167.3914.56. [DOI] [PubMed] [Google Scholar]
- Staples D. H., Hindley J., Billeter M. A., Weissmann C. Localization of Q-beta maturation cistron ribosome binding site. Nat New Biol. 1971 Sep 15;234(50):202–204. doi: 10.1038/newbio234202a0. [DOI] [PubMed] [Google Scholar]
- Staples D. H., Hindley J. Ribosome binding site of Q-beta RNA polymerase cistron. Nat New Biol. 1971 Sep 15;234(50):211–212. doi: 10.1038/newbio234211a0. [DOI] [PubMed] [Google Scholar]
- Steitz J. A. Discriminatory ribosome rebinding of isolated regions of protein synthesis initiation from the ribonucleic acid of bacteriophage R17. Proc Natl Acad Sci U S A. 1973 Sep;70(9):2605–2609. doi: 10.1073/pnas.70.9.2605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steitz J. A. Polypeptide chain initiation: nucleotide sequences of the three ribosomal binding sites in bacteriophage R17 RNA. Nature. 1969 Dec 6;224(5223):957–964. doi: 10.1038/224957a0. [DOI] [PubMed] [Google Scholar]
- Tai P. C., Wallace B. J., Davis B. D. Actions of aurintricarboxylate, kasugamycin, and pactamycin on Escherichia coli polysomes. Biochemistry. 1973 Feb;12(4):616–620. doi: 10.1021/bi00728a008. [DOI] [PubMed] [Google Scholar]
- Tinoco I., Jr, Uhlenbeck O. C., Levine M. D. Estimation of secondary structure in ribonucleic acids. Nature. 1971 Apr 9;230(5293):362–367. doi: 10.1038/230362a0. [DOI] [PubMed] [Google Scholar]
- Uhlenbeck O. C., Martin F. H., Doty P. Self-complementary oligoribonucleotides: effects of helix defects and guanylic acid-cytidylic acid base pairs. J Mol Biol. 1971 Apr 28;57(2):217–229. doi: 10.1016/0022-2836(71)90342-1. [DOI] [PubMed] [Google Scholar]
- Weiner A. M., Weber K. Natural read-through at the UGA termination signal of Q-beta coat protein cistron. Nat New Biol. 1971 Sep 15;234(50):206–209. doi: 10.1038/newbio234206a0. [DOI] [PubMed] [Google Scholar]
- de Wachter R., Merregaert J., Vandenberghe A., Contreras R., Fiers W. Studies on the bacteriophage MS2. The untranslated 5'-terminal nucleotide sequence preceding the first cistron. Eur J Biochem. 1971 Oct 14;22(3):400–414. doi: 10.1111/j.1432-1033.1971.tb01558.x. [DOI] [PubMed] [Google Scholar]


