Abstract
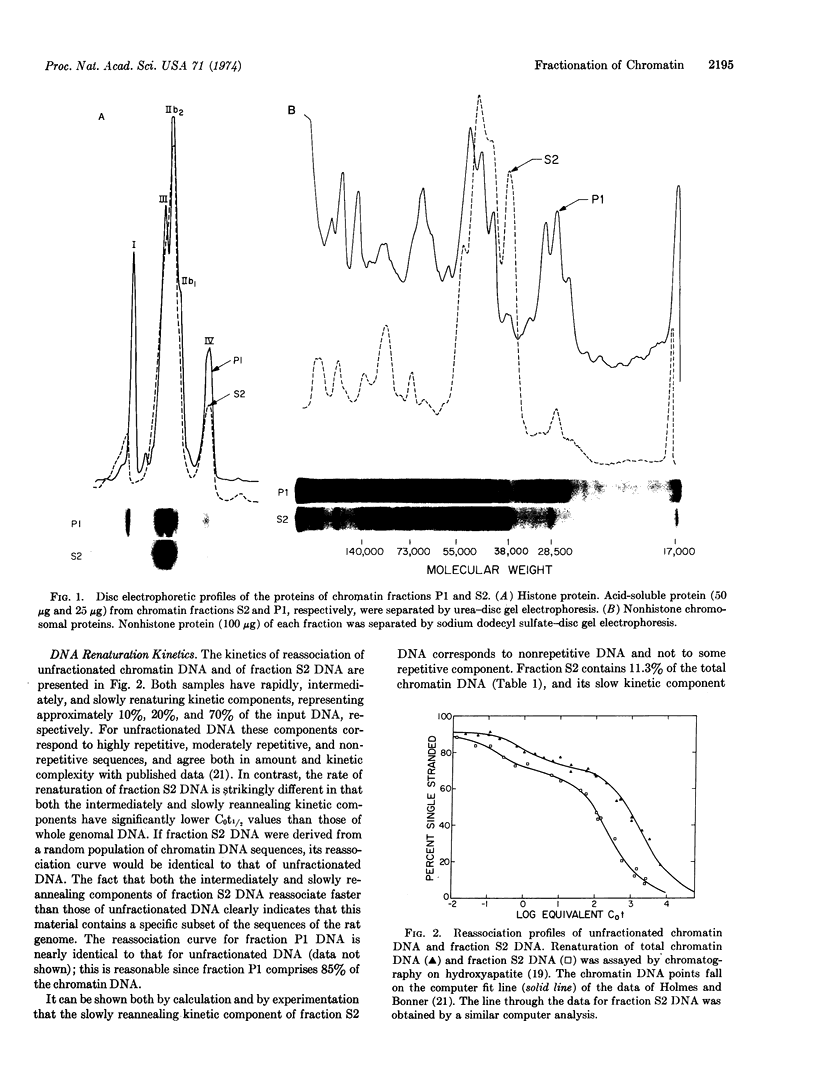
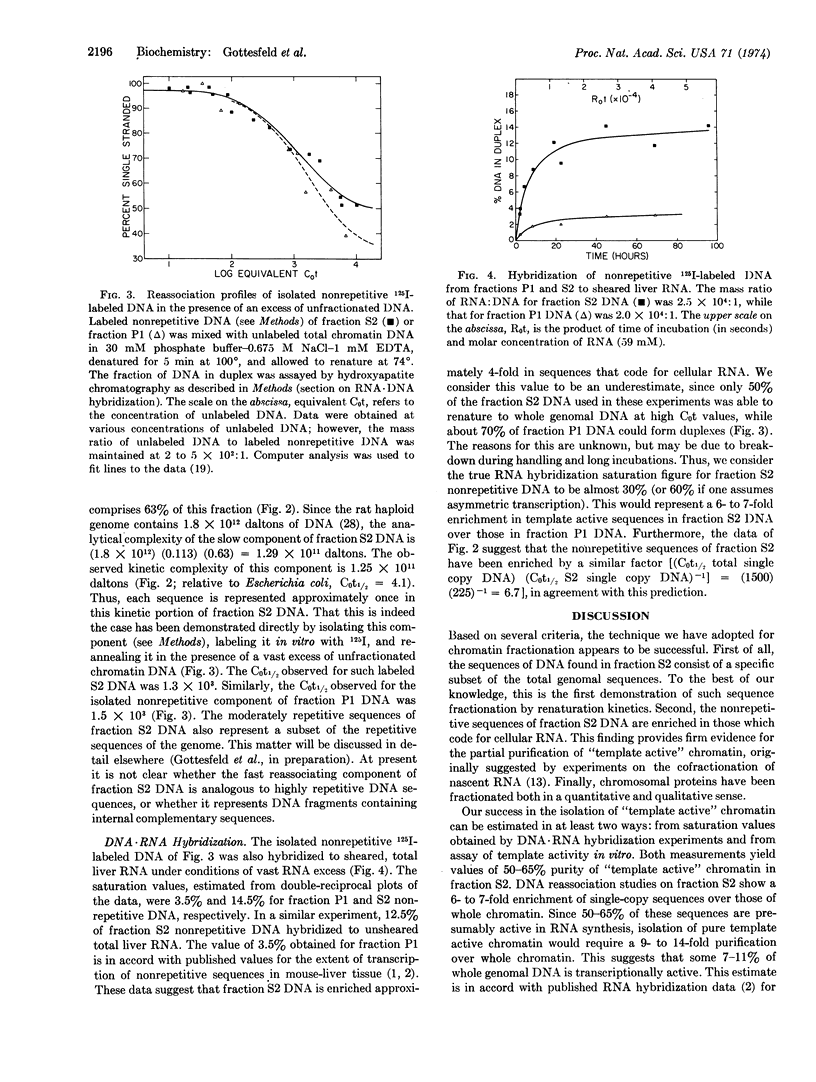
A fraction of rat-liver chromatin that is transcriptionally active in vivo has been purified 6- to 7-fold over whole chromatin. This was accomplished by selectively shearing chromatin with DNase II followed by fractionating the released portion on the basis of its solubility properties in 2 mM MgCl2. The resulting soluble material comprises 11% of the total chromatin DNA and is impoverished in histone and enriched in nonhistone protein. Compared with unsheared chromatin, this minor fraction exhibits marked differences in chromosomal protein species. DNA renaturation studies indicate that this fraction is composed of a specific subset of whole genomal DNA sequences. Furthermore, DNA·RNA hybridization experiments suggest that almost 60% of the nonrepetitious DNA sequences of this minor fraction could code for cellular RNA.
Keywords: chromatin fractionation, DNase II, DNA·RNA hybridization
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Axel R., Cedar H., Felsenfeld G. Synthesis of globin ribonucleic acid from duck-reticulocyte chromatin in vitro. Proc Natl Acad Sci U S A. 1973 Jul;70(7):2029–2032. doi: 10.1073/pnas.70.7.2029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Billing R. J., Bonner J. The structure of chromatin as revealed by deoxyribonuclease digestion studies. Biochim Biophys Acta. 1972 Oct 27;281(3):453–462. doi: 10.1016/0005-2787(72)90462-5. [DOI] [PubMed] [Google Scholar]
- Britten R. J., Davidson E. H. Repetitive and non-repetitive DNA sequences and a speculation on the origins of evolutionary novelty. Q Rev Biol. 1971 Jun;46(2):111–138. doi: 10.1086/406830. [DOI] [PubMed] [Google Scholar]
- Brown I. R., Church R. B. Transcription of nonrepeated DNA during mouse and rabbit development. Dev Biol. 1972 Sep;29(1):73–84. doi: 10.1016/0012-1606(72)90045-0. [DOI] [PubMed] [Google Scholar]
- Commerford S. L. Iodination of nucleic acids in vitro. Biochemistry. 1971 May 25;10(11):1993–2000. doi: 10.1021/bi00787a005. [DOI] [PubMed] [Google Scholar]
- FRENSTER J. H., ALLFREY V. G., MIRSKY A. E. REPRESSED AND ACTIVE CHROMATIN ISOLATED FROM INTERPHASE LYMPHOCYTES. Proc Natl Acad Sci U S A. 1963 Dec;50:1026–1032. doi: 10.1073/pnas.50.6.1026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilmour R. S., Paul J. Tissue-specific transcription of the globin gene in isolated chromatin. Proc Natl Acad Sci U S A. 1973 Dec;70(12):3440–3442. doi: 10.1073/pnas.70.12.3440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grouse L., Chilton M. D., McCarthy B. J. Hybridization of ribonucleic acid with unique sequences of mouse deoxyribonucleic acid. Biochemistry. 1972 Feb 29;11(5):798–805. doi: 10.1021/bi00755a019. [DOI] [PubMed] [Google Scholar]
- Lin P. P., Wilson R. F., Bonner J. Isolation and properties of nonhistone chromosomal proteins from pea chromatin. Mol Cell Biochem. 1973 Jun 27;1(2):197–207. doi: 10.1007/BF01659330. [DOI] [PubMed] [Google Scholar]
- Marushige K., Bonner J. Fractionation of liver chromatin. Proc Natl Acad Sci U S A. 1971 Dec;68(12):2941–2944. doi: 10.1073/pnas.68.12.2941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McConaughy B. L., McCarthy B. J. Fractionation of chromatin by thermal chromatography. Biochemistry. 1972 Mar 14;11(6):998–1003. doi: 10.1021/bi00756a008. [DOI] [PubMed] [Google Scholar]
- McConnell D. J., Bonner J. Preparation of highly purified ribonucleic acid polymerase; separation from polynucleotide phosphorylase and polyphosphate kinase. Biochemistry. 1972 Nov 7;11(23):4329–4336. doi: 10.1021/bi00773a020. [DOI] [PubMed] [Google Scholar]
- Moriyama Y., Hodnett J. L., Prestayko A. W., Busch H. Studies on the nuclear 4 to 7 s RNA of the Novikoff hepatoma. J Mol Biol. 1969 Jan;39(2):335–349. doi: 10.1016/0022-2836(69)90321-0. [DOI] [PubMed] [Google Scholar]
- Murphy E. C., Jr, Hall S. H., Shepherd J. H., Weiser R. S. Fractionation of mouse myeloma chromatin. Biochemistry. 1973 Sep 25;12(20):3843–3853. doi: 10.1021/bi00744a008. [DOI] [PubMed] [Google Scholar]
- Panyim S., Chalkley R. High resolution acrylamide gel electrophoresis of histones. Arch Biochem Biophys. 1969 Mar;130(1):337–346. doi: 10.1016/0003-9861(69)90042-3. [DOI] [PubMed] [Google Scholar]
- STUDIER F. W. SEDIMENTATION STUDIES OF THE SIZE AND SHAPE OF DNA. J Mol Biol. 1965 Feb;11:373–390. doi: 10.1016/s0022-2836(65)80064-x. [DOI] [PubMed] [Google Scholar]
- Simpson R. T., Reeck G. R. A comparison of the proteins of condensed and extended chromatin fractions of rabbit liver and calf thymus. Biochemistry. 1973 Sep 25;12(20):3853–3858. doi: 10.1021/bi00744a009. [DOI] [PubMed] [Google Scholar]