Abstract
On incubation of reticulocyte lysates at 30° in the absence of added hemin, protein synthesis declines sharply within 4-6 min, due to the action of a translational inhibitor. Partially purified preparations of this inhibitor, in concentrations that inhibit protein synthesis in the lysate, cause reduced binding of Met-tRNAfMet to derived 40S ribosomal subunits in a ribosomal-salt-wash-dependent assay system. Neither the association of salt wash proteins with the subunits nor the level of Met-tRNAfMet bound in preformed 40S complexes is reduced by the inhibitor. No Met-tRNAfMet deacylase activity could be detected in the inhibitor preparation. Protein synthesis in reticulocyte lysates lacking added hemin or containing exogenous inhibitor is maintained by addition of small amounts of an initiation preparation factor, “F-MP,” which may be involved in the binding of Met-tRNAfMet to 40S subunits. This binding constitutes a site for control of protein synthesis by hemin in reticulocytes.
Keywords: translation control, initiation factors, buoyant density gradients
Full text
PDF
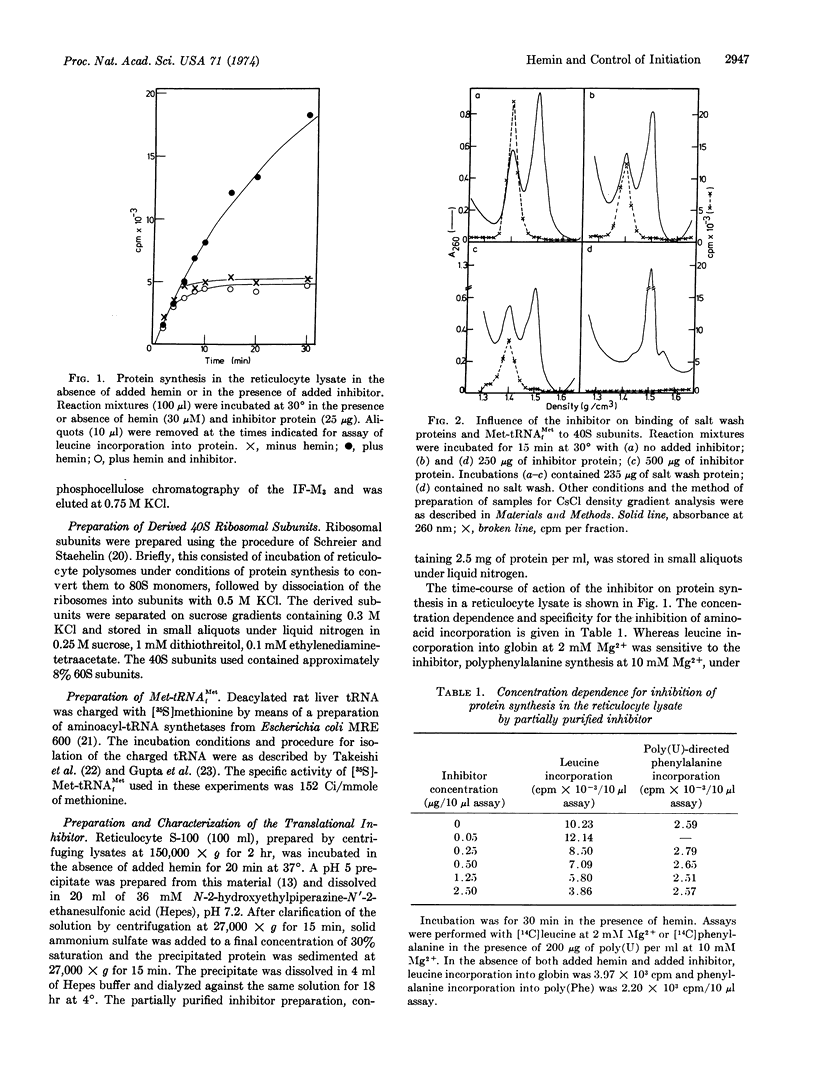
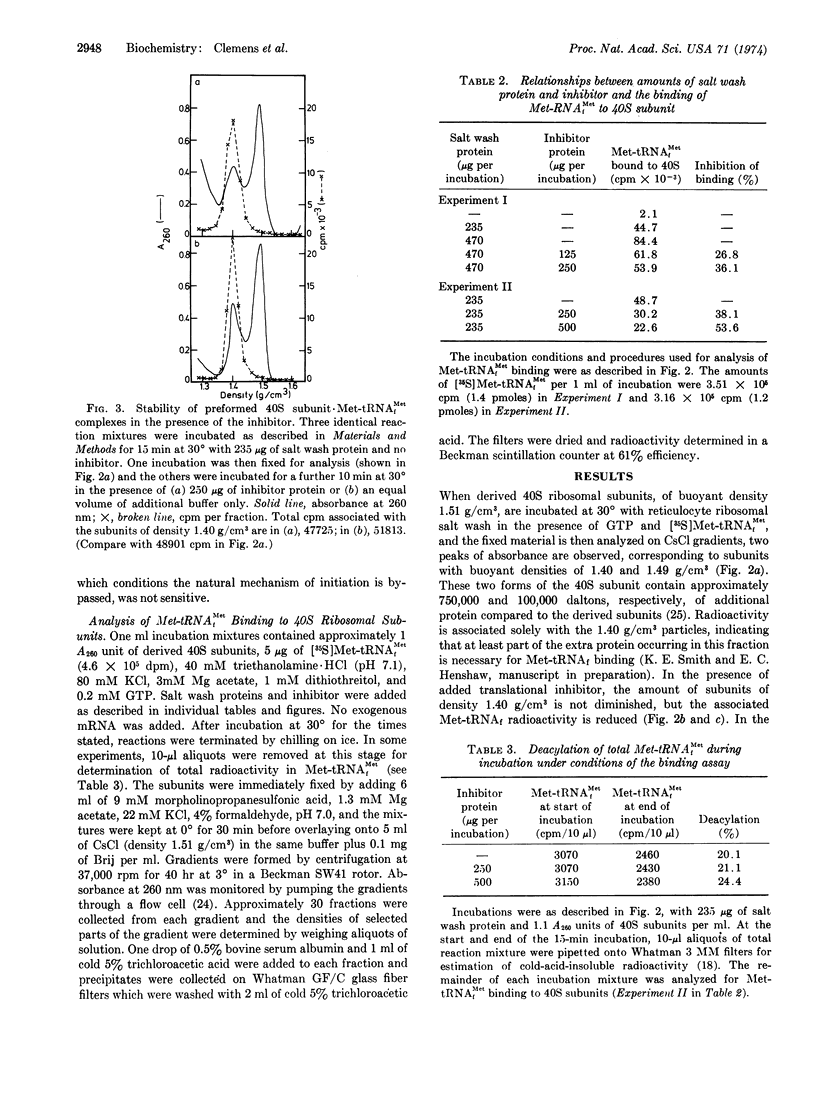
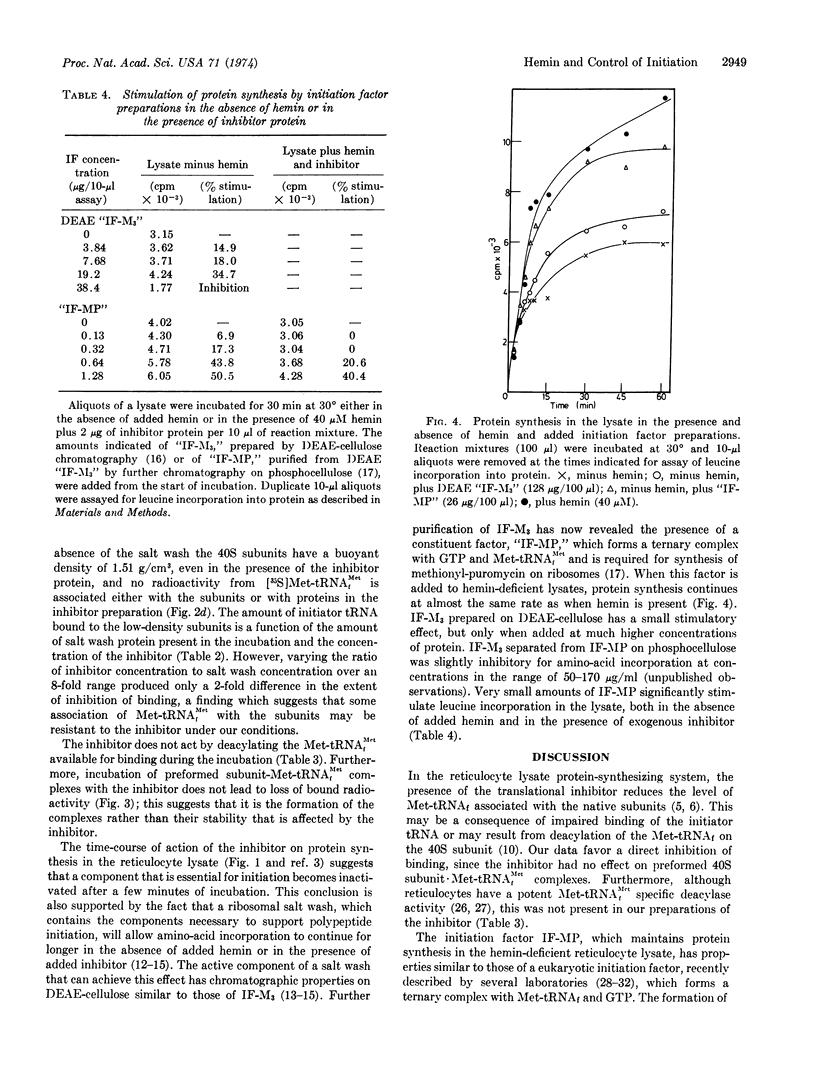

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adamson S. D., Yau P. M., Herbert E., Zucker W. V. Involvement of hemin, a stimulatory fraction from ribosomes and a protein synthesis inhibitor in the regulation of hemoglobin synthesis. J Mol Biol. 1972 Jan 28;63(2):247–264. doi: 10.1016/0022-2836(72)90373-7. [DOI] [PubMed] [Google Scholar]
- Ayuso-Parilla M., Henshaw E. C., Hirsch C. A. The ribosome cycle in mammalian protein synthesis. 3. Evidence that the nonribosomal proteins bound to the native smaller subunit are initiation factors. J Biol Chem. 1973 Jun 25;248(12):4386–4393. [PubMed] [Google Scholar]
- BRUNS G. P., LONDON I. M. THE EFFECT OF HEMIN ON THE SYNTHESIS OF GLOBIN. Biochem Biophys Res Commun. 1965 Jan 18;18:236–242. doi: 10.1016/0006-291x(65)90746-1. [DOI] [PubMed] [Google Scholar]
- Balkow K., Korner A. Stimulation of haemoglobin synthesis in reticulocyte lysates by initiation factors. FEBS Lett. 1971 Jan 12;12(3):157–160. doi: 10.1016/0014-5793(71)80057-1. [DOI] [PubMed] [Google Scholar]
- Balkow K., Mizuno S., Fisher J. M., Rabinovitz M. Hemin control of globin synthesis: effect of a translational repressor on Met-tRNAf binding to the small ribosomal subunit and its relation to the activity and alailability of an initiation factor. Biochim Biophys Acta. 1973 Oct 26;324(3):397–409. doi: 10.1016/0005-2787(73)90284-0. [DOI] [PubMed] [Google Scholar]
- Balkow K., Mizuno S., Rabinovitz M. Inhibition of an initiation codon function by hemin deficiency and the hemin-controlled translational repressor in the reticulocyte cell-free system. Biochem Biophys Res Commun. 1973 Sep 5;54(1):315–323. doi: 10.1016/0006-291x(73)90925-x. [DOI] [PubMed] [Google Scholar]
- Beuzard Y., London I. M. The effects of hemin and double-stranded RNA on alpha and beta globin synthesis in reticulocyte and Krebs II ascites cell-free systems and the relationship of these effects to an initiation factor preparation. Proc Natl Acad Sci U S A. 1974 Jul;71(7):2863–2866. doi: 10.1073/pnas.71.7.2863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beuzard Y., Rodvien R., London I. M. Effect of hemin on the synthesis of hemoglobin and other proteins in mammalian cells. Proc Natl Acad Sci U S A. 1973 Apr;70(4):1022–1026. doi: 10.1073/pnas.70.4.1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cashion L. M., Stanley W. M., Jr Comparative studies on the properties of the eukaryotic protein synthesis initiation factor IF-I from several sources. Biochim Biophys Acta. 1973 Oct 26;324(3):410–419. doi: 10.1016/0005-2787(73)90285-2. [DOI] [PubMed] [Google Scholar]
- Cashion L. M., Stanley W. M., Jr Two eukaryotic initiation factors (IF-I and IF-II) of protein synthesis that are required to form an initiation complex with rabbit reticulocyte ribosomes. Proc Natl Acad Sci U S A. 1974 Feb;71(2):436–440. doi: 10.1073/pnas.71.2.436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y. C., Woodley C. L., Bose K. K., Gupta K. K. Protein synthesis in rabbit reticulocytes: characteristics of a Met-tRNA Met f binding factor. Biochem Biophys Res Commun. 1972 Jul 11;48(1):1–9. doi: 10.1016/0006-291x(72)90335-x. [DOI] [PubMed] [Google Scholar]
- Dettman G. L., Stanley W. M., Jr Recognition of eukaryotic initiator tRNA by an initiation factor and the transfer of the methionine moiety into peptide linkage. Biochim Biophys Acta. 1972 Nov 16;287(1):124–133. doi: 10.1016/0005-2787(72)90336-x. [DOI] [PubMed] [Google Scholar]
- Gross M., Rabinovitz M. Control of globin synthesis by hemin: factors influencing formation of an inhibitor of globin chain initiation in reticulocyte lysates. Biochim Biophys Acta. 1972 Dec 6;287(2):340–352. doi: 10.1016/0005-2787(72)90383-8. [DOI] [PubMed] [Google Scholar]
- Gross M., Rabinovitz M. Control of globin synthesis in cell-free preparations of reticulocytes by formation of a translational repressor that is inactivated by hemin. Proc Natl Acad Sci U S A. 1972 Jun;69(6):1565–1568. doi: 10.1073/pnas.69.6.1565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta N. K., Aerni R. J. Protein synthesis in rabbit reticulocytes: control of protein synthesis initiation by a met-tRNA Met f deacylase and peptide chain initiation factors. Biochem Biophys Res Commun. 1973 Apr 16;51(4):907–916. doi: 10.1016/0006-291x(73)90013-2. [DOI] [PubMed] [Google Scholar]
- Gupta N., Chatterjee N. K., Woodley C. L., Bose K. K. Protein synthesis in rabbit reticulocytes. Factors controlling internal and terminal methionine codon recognition by the methionyl transfer ribonucleic acid species. J Biol Chem. 1971 Dec 25;246(24):7460–7469. [PubMed] [Google Scholar]
- Hirsch C. A., Cox M. A., van Venrooij W. J., Henshaw E. C. The ribosome cycle in mammalian protein synthesis. II. Association of the native smaller ribosomal subunit with protein factors. J Biol Chem. 1973 Jun 25;248(12):4377–4385. [PubMed] [Google Scholar]
- Hunt T., Vanderhoff G., London I. M. Control of globin synthesis: the role of heme. J Mol Biol. 1972 May 28;66(3):471–481. doi: 10.1016/0022-2836(72)90427-5. [DOI] [PubMed] [Google Scholar]
- Kaempfer R., Kaufman J. Translational control of hemoglobin synthesis by an initiation factor required for recycling of ribosomes and for their binding to messenger RNA. Proc Natl Acad Sci U S A. 1972 Nov;69(11):3317–3321. doi: 10.1073/pnas.69.11.3317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Legon S., Jackson R. J., Hunt T. Control of protein synthesis in reticulocyte lysates by haemin. Nat New Biol. 1973 Jan 31;241(109):150–152. doi: 10.1038/newbio241150a0. [DOI] [PubMed] [Google Scholar]
- Levin D. H., Kyner D., Acs G. Protein initiation in eukaryotes: formation and function of a ternary complex composed of a partially purified ribosomal factor, methionyl transfer RNA, and guanosine triphosphate. Proc Natl Acad Sci U S A. 1973 Jan;70(1):41–45. doi: 10.1073/pnas.70.1.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin D. H., Kyner D., Acs G. Protein synthesis initiation in eukaryotes. Characterization of ribosomal factors from mouse fibroblasts. J Biol Chem. 1973 Sep 25;248(18):6416–6425. [PubMed] [Google Scholar]
- Maxwell C. R., Kamper C. S., Rabinovitz M. Hemin control of globin synthesis: an assay for the inhibitor formed in the absence of hemin and some characteristics of its formation. J Mol Biol. 1971 May 28;58(1):317–327. doi: 10.1016/0022-2836(71)90249-x. [DOI] [PubMed] [Google Scholar]
- Mizuno S., Fisher J. M., Rabinovitz M. Hemin control of globin synthesis: action of an inhibitor formed in the absence of hemin on the reticulocyte cell-free system and its reversal by a ribosomal factor. Biochim Biophys Acta. 1972 Jul 31;272(4):638–650. [PubMed] [Google Scholar]
- Morrisey J., Hardesty B. Met-tRNA hydrolase from reticulocytes specific for Met-tRNA f Met on 40S ribosomal subunits. Arch Biochem Biophys. 1972 Sep;152(1):385–397. doi: 10.1016/0003-9861(72)90228-7. [DOI] [PubMed] [Google Scholar]
- Morton B. E., Hirsch C. A. A high-resolution system for gradient analysis. Anal Biochem. 1970 Apr;34(2):544–559. doi: 10.1016/0003-2697(70)90140-5. [DOI] [PubMed] [Google Scholar]
- Prichard P. M., Picciano D. J., Laycock D. G., Anderson W. F. Translation of exogenous messenger RNA for hemoglobin on reticulocyte and liver ribosomes. Proc Natl Acad Sci U S A. 1971 Nov;68(11):2752–2756. doi: 10.1073/pnas.68.11.2752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RajBhandary U. L., Ghosh H. P. Studies on polynucleotides. XCI. Yeast methionine transfer ribonucleic acid: purification, properties, and terminal nucleotide sequences. J Biol Chem. 1969 Mar 10;244(5):1104–1113. [PubMed] [Google Scholar]
- Schreier M. H., Staehelin T. Initiation of eukaryotic protein synthesis: (Met-tRNA f -40S ribosome) initiation complex catalysed by purified initiation factors in the absence of mRNA. Nat New Biol. 1973 Mar 14;242(115):35–38. doi: 10.1038/newbio242035a0. [DOI] [PubMed] [Google Scholar]
- Schreier M. H., Staehelin T. Initiation of mammalian protein synthesis: the importance of ribosome and initiation factor quality for the efficiency of in vitro systems. J Mol Biol. 1973 Feb 19;73(3):329–349. doi: 10.1016/0022-2836(73)90346-x. [DOI] [PubMed] [Google Scholar]
- Shafritz D. A., Anderson W. F. Isolation and partial characterization of reticulocyte factors M1 and M2. J Biol Chem. 1970 Nov 10;245(21):5553–5559. [PubMed] [Google Scholar]
- Takeishi K., Ukita T., Nishimura S. Characterization of two species of methionine transfer ribonucleic acid from bakers' yeast. J Biol Chem. 1968 Nov 10;243(21):5761–5768. [PubMed] [Google Scholar]
- Zucker W. V., Schulman H. M. Stimulation of globin-chain initiation by hemin in the reticulocyte cell-free system. Proc Natl Acad Sci U S A. 1968 Feb;59(2):582–589. doi: 10.1073/pnas.59.2.582. [DOI] [PMC free article] [PubMed] [Google Scholar]


