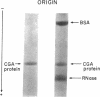
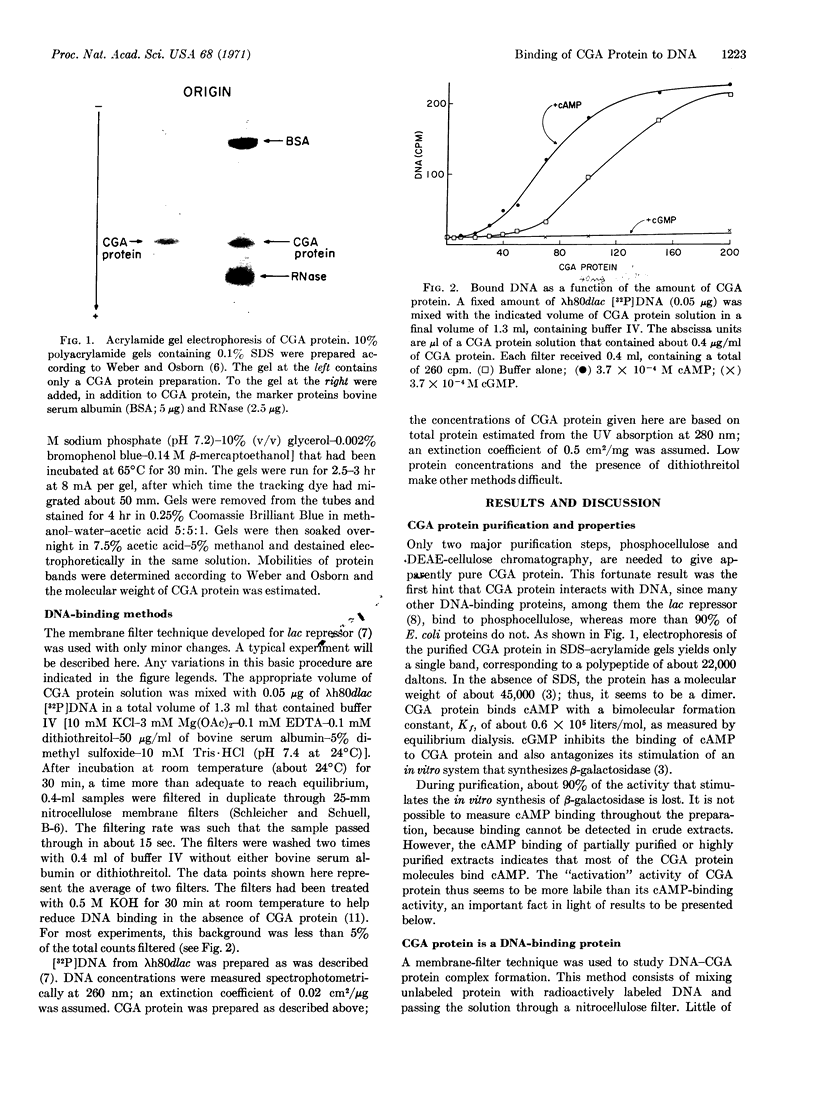
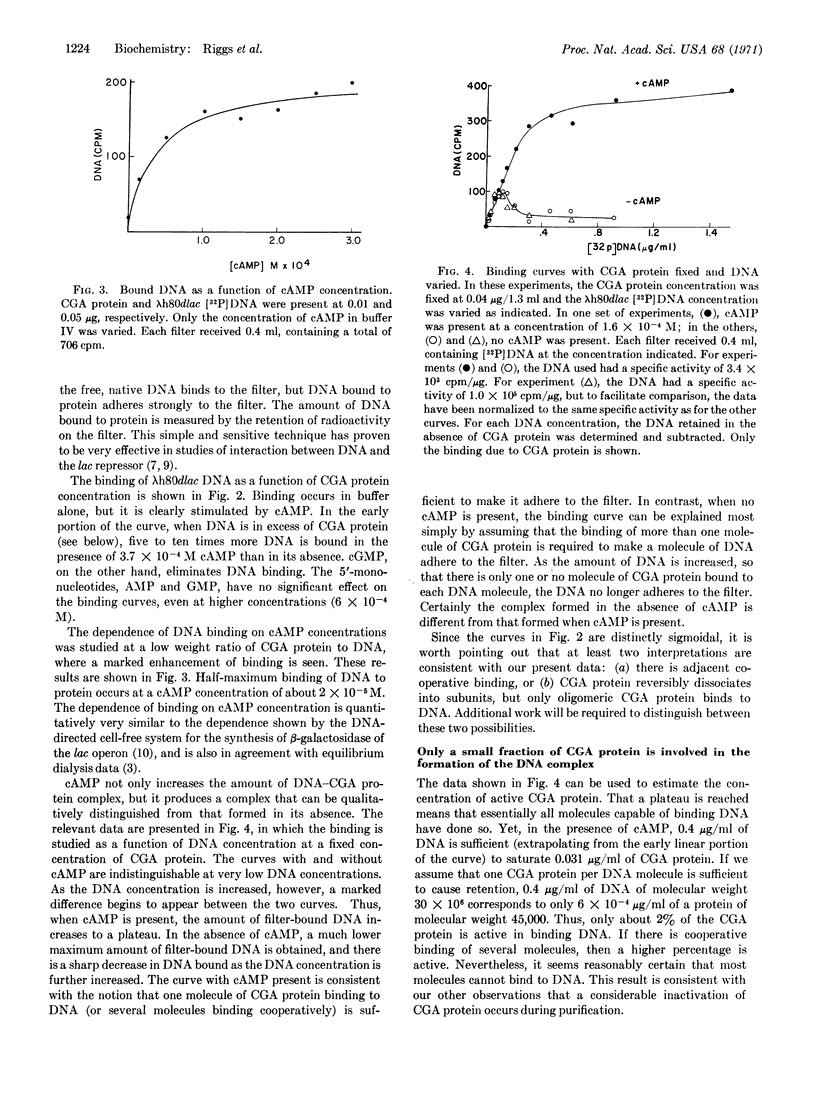
Abstract
A protein required for the activation of the lac operon has been extensively purified and partly characterized. This protein, called CGA protein (catabolite gene activator protein, sometimes named CAP), is a dimer with subunits of 22,000 daltons. Purified CGA protein has a substantial affinity for DNA; this affinity is greatly strengthened by cAMP and strongly inhibited by cGMP. Other studies have shown that these cyclic nucleotides compete for a binding site on CGA protein. The opposing effects of the two cyclic compounds in DNA-CGA protein binding show a parallel behavior to their effects on the expression of the lac operon. Thus cAMP, in addition to CGA protein, is required for expression of the lac operon, whereas cGMP inhibits the expression. The obvious inference is that CGA protein activates the lac operon by binding to the DNA under the influence of cAMP. Thus, CGA protein seems to be a new type of regulatory protein: a DNA-binding activator.
Keywords: phosphocellulose, DEAE-cellulose, lac operon DNA, cyclic AMP
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Burgess R. R., Travers A. A., Dunn J. J., Bautz E. K. Factor stimulating transcription by RNA polymerase. Nature. 1969 Jan 4;221(5175):43–46. doi: 10.1038/221043a0. [DOI] [PubMed] [Google Scholar]
- Gilbert W., Müller-Hill B. The lac operator is DNA. Proc Natl Acad Sci U S A. 1967 Dec;58(6):2415–2421. doi: 10.1073/pnas.58.6.2415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MAGASANIK B. Catabolite repression. Cold Spring Harb Symp Quant Biol. 1961;26:249–256. doi: 10.1101/sqb.1961.026.01.031. [DOI] [PubMed] [Google Scholar]
- Pastan I., Perlman R. Cyclic adenosine monophosphate in bacteria. Science. 1970 Jul 24;169(3943):339–344. doi: 10.1126/science.169.3943.339. [DOI] [PubMed] [Google Scholar]
- Pirrotta V., Ptashne M. Isolation of the 434 phage repressor. Nature. 1969 May 10;222(5193):541–544. doi: 10.1038/222541a0. [DOI] [PubMed] [Google Scholar]
- Riggs A. D., Bourgeois S. On the assay, isolation and characterization of the lac repressor. J Mol Biol. 1968 Jul 14;34(2):361–364. doi: 10.1016/0022-2836(68)90260-x. [DOI] [PubMed] [Google Scholar]
- Riggs A. D., Newby R. F., Bourgeois S. lac repressor--operator interaction. II. Effect of galactosides and other ligands. J Mol Biol. 1970 Jul 28;51(2):303–314. doi: 10.1016/0022-2836(70)90144-0. [DOI] [PubMed] [Google Scholar]
- Riggs A. D., Suzuki H., Bourgeois S. Lac repressor-operator interaction. I. Equilibrium studies. J Mol Biol. 1970 Feb 28;48(1):67–83. doi: 10.1016/0022-2836(70)90219-6. [DOI] [PubMed] [Google Scholar]
- Silverstone A. E., Arditti R. R., Magasanik B. Catabolite-insensitive revertants of lac promoter mutants. Proc Natl Acad Sci U S A. 1970 Jul;66(3):773–779. doi: 10.1073/pnas.66.3.773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber K., Osborn M. The reliability of molecular weight determinations by dodecyl sulfate-polyacrylamide gel electrophoresis. J Biol Chem. 1969 Aug 25;244(16):4406–4412. [PubMed] [Google Scholar]
- Zubay G., Schwartz D., Beckwith J. Mechanism of activation of catabolite-sensitive genes: a positive control system. Proc Natl Acad Sci U S A. 1970 May;66(1):104–110. doi: 10.1073/pnas.66.1.104. [DOI] [PMC free article] [PubMed] [Google Scholar]