Abstract
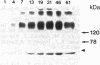
Newly synthesized class II HLA antigens being transported to the surface of human B-lymphoblastoid cell lines (B-LCL) interact with transferrin-neuraminidase conjugates internalized by means of receptor-mediated endocytosis. Class II antigens, isolated from [35S]methionine-labeled B-LCL after incubation with the conjugates at 37 degrees C, showed extensive desialylation of associated invariant chain and detectable loss of beta-subunit sialic acid on analysis by two-dimensional gel electrophoresis. An equal amount of unconjugated neuraminidase had no effect, and desialylation of class II antigen components was blocked when access of transferrin-neuraminidase conjugates to the B-LCL transferrin receptors was competitively inhibited by the addition of excess iron-saturated transferrin. The conjugates were shown to cycle through the cells in the same way as unconjugated transferrin, being first internalized and then rapidly secreted in an undegraded form. The data suggest that the exocytic pathway taken by class II antigens intersects the route followed by recycling transferrin receptors and that the interaction occurs prior to the dissociation of the invariant chain from the class II antigen complex. Similar intracellular interactions between class II molecules and foreign proteins internalized by antigen-presenting cells may be important in class II antigen-restricted recognition by helper T lymphocytes.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Accolla R. S., Carra G., Buchegger F., Carrel S., Mach J. P. The human Ia-associated invariant chain is synthesized in Ia-negative B cell variants and is not expressed on the cell surface of both Ia-negative and Ia-positive parental cells. J Immunol. 1985 May;134(5):3265–3271. [PubMed] [Google Scholar]
- Carlsson J., Drevin H., Axén R. Protein thiolation and reversible protein-protein conjugation. N-Succinimidyl 3-(2-pyridyldithio)propionate, a new heterobifunctional reagent. Biochem J. 1978 Sep 1;173(3):723–737. doi: 10.1042/bj1730723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charron D. J., McDevitt H. O. Analysis of HLA-D region-associated molecules with monoclonal antibody. Proc Natl Acad Sci U S A. 1979 Dec;76(12):6567–6571. doi: 10.1073/pnas.76.12.6567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chesnut R. W., Colon S. M., Grey H. M. Requirements for the processing of antigens by antigen-presenting B cells. I. Functional comparison of B cell tumors and macrophages. J Immunol. 1982 Dec;129(6):2382–2388. [PubMed] [Google Scholar]
- Claesson L., Peterson P. A. Association of human gamma chain with class II transplantation antigens during intracellular transport. Biochemistry. 1983 Jun 21;22(13):3206–3213. doi: 10.1021/bi00282a026. [DOI] [PubMed] [Google Scholar]
- Dautry-Varsat A., Ciechanover A., Lodish H. F. pH and the recycling of transferrin during receptor-mediated endocytosis. Proc Natl Acad Sci U S A. 1983 Apr;80(8):2258–2262. doi: 10.1073/pnas.80.8.2258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geuze H. J., Slot J. W., Strous G. J., Lodish H. F., Schwartz A. L. Intracellular site of asialoglycoprotein receptor-ligand uncoupling: double-label immunoelectron microscopy during receptor-mediated endocytosis. Cell. 1983 Jan;32(1):277–287. doi: 10.1016/0092-8674(83)90518-4. [DOI] [PubMed] [Google Scholar]
- Goldstein J. L., Anderson R. G., Brown M. S. Coated pits, coated vesicles, and receptor-mediated endocytosis. Nature. 1979 Jun 21;279(5715):679–685. doi: 10.1038/279679a0. [DOI] [PubMed] [Google Scholar]
- Guy K., Van Heyningen V., Cohen B. B., Deane D. L., Steel C. M. Differential expression and serologically distinct subpopulations of human Ia antigens detected with monoclonal antibodies to Ia alpha and beta chains. Eur J Immunol. 1982 Nov;12(11):942–948. doi: 10.1002/eji.1830121109. [DOI] [PubMed] [Google Scholar]
- Jones P. P., Murphy D. B., Hewgill D., McDevitt H. O. Detection of a common polypeptide chain in I--A and I--E sub-region immunoprecipitates. Mol Immunol. 1979 Jan;16(1):51–60. doi: 10.1016/0161-5890(79)90027-0. [DOI] [PubMed] [Google Scholar]
- Karin M., Mintz B. Receptor-mediated endocytosis of transferrin in developmentally totipotent mouse teratocarcinoma stem cells. J Biol Chem. 1981 Apr 10;256(7):3245–3252. [PubMed] [Google Scholar]
- Klausner R. D., Ashwell G., van Renswoude J., Harford J. B., Bridges K. R. Binding of apotransferrin to K562 cells: explanation of the transferrin cycle. Proc Natl Acad Sci U S A. 1983 Apr;80(8):2263–2266. doi: 10.1073/pnas.80.8.2263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kvist S., Wiman K., Claesson L., Peterson P. A., Dobberstein B. Membrane insertion and oligomeric assembly of HLA-DR histocompatibility antigens. Cell. 1982 May;29(1):61–69. doi: 10.1016/0092-8674(82)90090-3. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lanzavecchia A. Antigen-specific interaction between T and B cells. Nature. 1985 Apr 11;314(6011):537–539. doi: 10.1038/314537a0. [DOI] [PubMed] [Google Scholar]
- Larrick J. W., Cresswell P. Transferrin receptors on human B and T lymphoblastoid cell lines. Biochim Biophys Acta. 1979 Apr 3;583(4):483–490. doi: 10.1016/0304-4165(79)90065-5. [DOI] [PubMed] [Google Scholar]
- Machamer C. E., Cresswell P. Biosynthesis and glycosylation of the invariant chain associated with HLA-DR antigens. J Immunol. 1982 Dec;129(6):2564–2569. [PubMed] [Google Scholar]
- Machamer C. E., Cresswell P. Monensin prevents terminal glycosylation of the N- and O-linked oligosaccharides of the HLA-DR-associated invariant chain and inhibits its dissociation from the alpha-beta chain complex. Proc Natl Acad Sci U S A. 1984 Mar;81(5):1287–1291. doi: 10.1073/pnas.81.5.1287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Farrell P. Z., Goodman H. M., O'Farrell P. H. High resolution two-dimensional electrophoresis of basic as well as acidic proteins. Cell. 1977 Dec;12(4):1133–1141. doi: 10.1016/0092-8674(77)90176-3. [DOI] [PubMed] [Google Scholar]
- Octave J. N., Schneider Y. J., Crichton R. R., Trouet A. Transferrin uptake by cultured rat embryo fibroblasts. The influence of temperature and incubation time, subcellular distribution and short-term kinetic studies. Eur J Biochem. 1981 Apr;115(3):611–618. [PubMed] [Google Scholar]
- Owen M. J., Kissonerghis A. M., Lodish H. F., Crumpton M. J. Biosynthesis and maturation of HLA-DR antigens in vivo. J Biol Chem. 1981 Sep 10;256(17):8987–8993. [PubMed] [Google Scholar]
- Pletscher M., Pernis B. Internalized membrane immunoglobulin meets intracytoplasmic DR antigen in human B lymphoblastoid cells. Eur J Immunol. 1983 Jul;13(7):581–584. doi: 10.1002/eji.1830130713. [DOI] [PubMed] [Google Scholar]
- Quaranta V., Majdic O., Stingl G., Liszka K., Honigsmann H., Knapp W. A human Ia cytoplasmic determinant located on multiple forms of invariant chain (gamma, gamma 2, gamma 3). J Immunol. 1984 Apr;132(4):1900–1905. [PubMed] [Google Scholar]
- Rothman J. E. The golgi apparatus: two organelles in tandem. Science. 1981 Sep 11;213(4513):1212–1219. doi: 10.1126/science.7268428. [DOI] [PubMed] [Google Scholar]
- Snider M. D., Rogers O. C. Intracellular movement of cell surface receptors after endocytosis: resialylation of asialo-transferrin receptor in human erythroleukemia cells. J Cell Biol. 1985 Mar;100(3):826–834. doi: 10.1083/jcb.100.3.826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein B. S., Bensch K. G., Sussman H. H. Complete inhibition of transferrin recycling by monensin in K562 cells. J Biol Chem. 1984 Dec 10;259(23):14762–14772. [PubMed] [Google Scholar]
- Streicher H. Z., Berkower I. J., Busch M., Gurd F. R., Berzofsky J. A. Antigen conformation determines processing requirements for T-cell activation. Proc Natl Acad Sci U S A. 1984 Nov;81(21):6831–6835. doi: 10.1073/pnas.81.21.6831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tartakoff A. M. Perturbation of vesicular traffic with the carboxylic ionophore monensin. Cell. 1983 Apr;32(4):1026–1028. doi: 10.1016/0092-8674(83)90286-6. [DOI] [PubMed] [Google Scholar]
- Tycko B., Maxfield F. R. Rapid acidification of endocytic vesicles containing alpha 2-macroglobulin. Cell. 1982 Mar;28(3):643–651. doi: 10.1016/0092-8674(82)90219-7. [DOI] [PubMed] [Google Scholar]
- Unanue E. R. Antigen-presenting function of the macrophage. Annu Rev Immunol. 1984;2:395–428. doi: 10.1146/annurev.iy.02.040184.002143. [DOI] [PubMed] [Google Scholar]
- WARREN L. The thiobarbituric acid assay of sialic acids. J Biol Chem. 1959 Aug;234(8):1971–1975. [PubMed] [Google Scholar]
- Willingham M. C., Hanover J. A., Dickson R. B., Pastan I. Morphologic characterization of the pathway of transferrin endocytosis and recycling in human KB cells. Proc Natl Acad Sci U S A. 1984 Jan;81(1):175–179. doi: 10.1073/pnas.81.1.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willingham M. C., Pastan I. H. Transit of epidermal growth factor through coated pits of the Golgi system. J Cell Biol. 1982 Jul;94(1):207–212. doi: 10.1083/jcb.94.1.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willingham M. C., Pastan I. The receptosome: an intermediate organelle of receptor mediated endocytosis in cultured fibroblasts. Cell. 1980 Aug;21(1):67–77. doi: 10.1016/0092-8674(80)90115-4. [DOI] [PubMed] [Google Scholar]
- Yamashiro D. J., Tycko B., Fluss S. R., Maxfield F. R. Segregation of transferrin to a mildly acidic (pH 6.5) para-Golgi compartment in the recycling pathway. Cell. 1984 Jul;37(3):789–800. doi: 10.1016/0092-8674(84)90414-8. [DOI] [PubMed] [Google Scholar]