Abstract
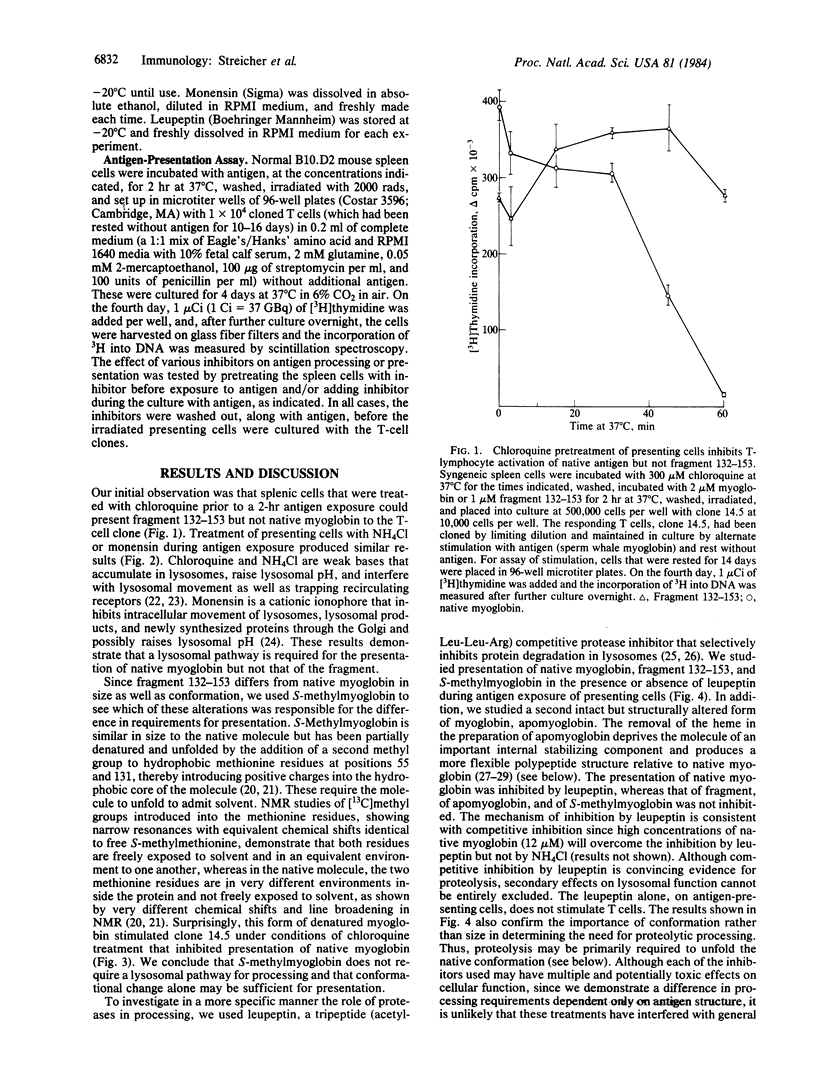
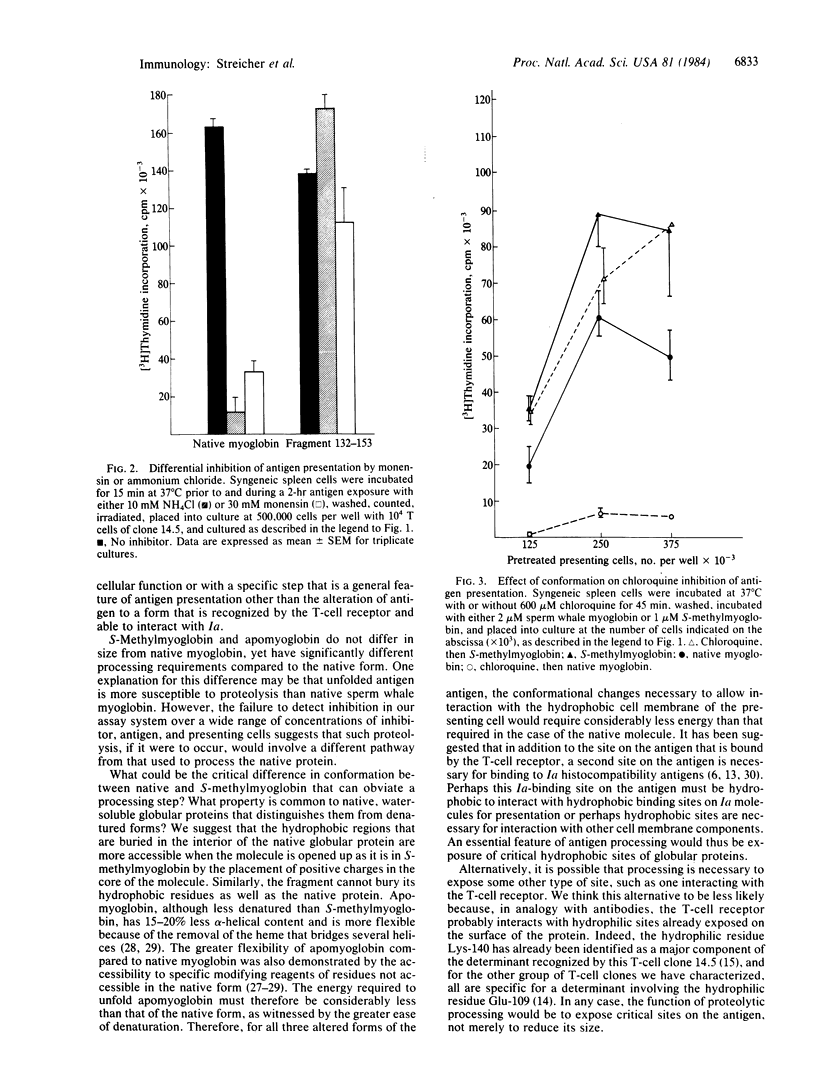
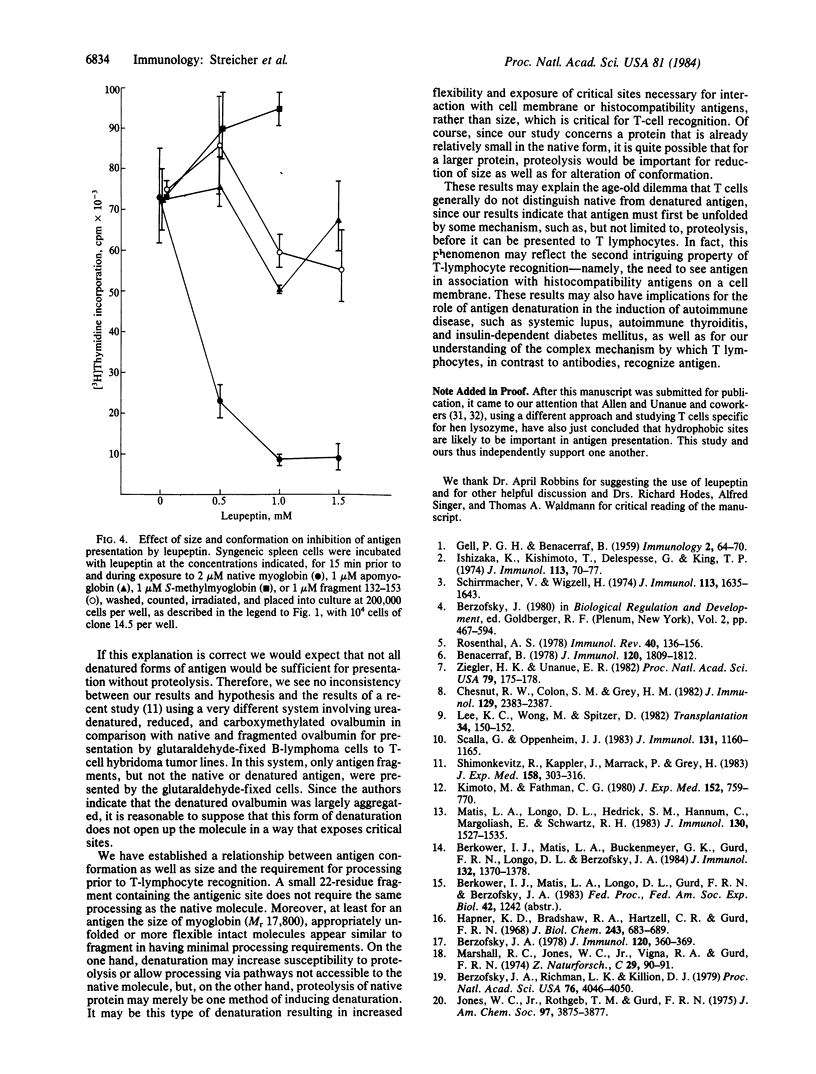
We studied the difference in requirements for processing and presentation to a single T-cell clone of four different forms of the same epitope of sperm whale myoglobin--namely, on the native protein, on two conformationally altered forms of the protein, or as a 22-residue antigenic peptide fragment. The T-cell clone was I-Ed-restricted and specific for an epitope on the CNBr fragment 132-153 involving Lys-140. As inhibitors of macrophage processing of antigen, we used several agents that inhibit lysosomal function: the weak bases chloroquine and NH4Cl, the cationic ionophore monensin, and the competitive protease inhibitor leupeptin. When these agents were used to inhibit processing of antigen by presenting cells and then washed out before T cells were added to culture, they inhibited the presentation of native antigen but not of fragment 132-153. To our surprise, the intact but denatured form, S-methylmyoglobin, behaved like the fragment not like the native protein. Apomyoglobin was intermediate in susceptibility to inhibition. Thus, native myoglobin requires a processing step that appears to involve lysosomal proteolysis, which is not required by fragment 132-153 or the denatured unfolded forms. For an antigen the size of myoglobin (Mr 17,800), it appears that unfolding of the native conformation, rather than further reduction in size, is the critical parameter determining the need for processing. Since a major difference between native myoglobin and the other forms is the greater accessibility in the latter of sites, such as hydrophobic residues, buried in the native protein, we propose that processing may be necessary to expose these sites, perhaps for interaction with the cell membrane or the Ia of the antigen-presenting cell.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allen P. M., Strydom D. J., Unanue E. R. Processing of lysozyme by macrophages: identification of the determinant recognized by two T-cell hybridomas. Proc Natl Acad Sci U S A. 1984 Apr;81(8):2489–2493. doi: 10.1073/pnas.81.8.2489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen P. M., Unanue E. R. Differential requirements for antigen processing by macrophages for lysozyme-specific T cell hybridomas. J Immunol. 1984 Mar;132(3):1077–1079. [PubMed] [Google Scholar]
- Aoyagi T., Umezawa H. The relationships between enzyme inhibitors and function of mammalian cells. Acta Biol Med Ger. 1981;40(10-11):1523–1529. [PubMed] [Google Scholar]
- BRESLOW E., BEYCHOK S., HARDMAN K. D., GURD F. R. RELATIVE CONFORMATIONS OF SPERM WHALE METMYOGLOBIN AND APOMYOGLOBIN IN SOLUTION. J Biol Chem. 1965 Jan;240:304–309. [PubMed] [Google Scholar]
- BRESLOW E. CHANGES IN SIDE CHAIN REACTIVITY ACCOMPANYING THE BINDING OF HEME TO SPERM WHALE APOMYOGLOBIN. J Biol Chem. 1964 Feb;239:486–496. [PubMed] [Google Scholar]
- Basu S. K., Goldstein J. L., Anderson R. G., Brown M. S. Monensin interrupts the recycling of low density lipoprotein receptors in human fibroblasts. Cell. 1981 May;24(2):493–502. doi: 10.1016/0092-8674(81)90340-8. [DOI] [PubMed] [Google Scholar]
- Benacerraf B. A hypothesis to relate the specificity of T lymphocytes and the activity of I region-specific Ir genes in macrophages and B lymphocytes. J Immunol. 1978 Jun;120(6):1809–1812. [PubMed] [Google Scholar]
- Berkower I., Matis L. A., Buckenmeyer G. K., Gurd F. R., Longo D. L., Berzofsky J. A. Identification of distinct predominant epitopes recognized by myoglobin-specific T cells under the control of different Ir genes and characterization of representative T cell clones. J Immunol. 1984 Mar;132(3):1370–1378. [PubMed] [Google Scholar]
- Berzofsky J. A. Genetic control of the immune response to mammalian myoglobins in mice I. More than one I-region gene in H-2 controls the antibody response. J Immunol. 1978 Feb;120(2):360–369. [PubMed] [Google Scholar]
- Berzofsky J. A., Richman L. K., Killion D. J. Distinct H-2-linked Ir genes control both antibody and T cell responses to different determinants on the same antigen, myoglobin. Proc Natl Acad Sci U S A. 1979 Aug;76(8):4046–4050. doi: 10.1073/pnas.76.8.4046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GELL P. G., BENACERRAF B. Studies on hypersensitivity. II. Delayed hypersensitivity to denatured proteins in guinea pigs. Immunology. 1959 Jan;2(1):64–70. [PMC free article] [PubMed] [Google Scholar]
- Grinde B., Seglen P. O. Differential effects of proteinase inhibitors and amines on the lysosomal and non-lysosomal pathways of protein degradation in isolated rat hepatocytes. Biochim Biophys Acta. 1980 Sep 17;632(1):73–86. doi: 10.1016/0304-4165(80)90250-0. [DOI] [PubMed] [Google Scholar]
- HARRISON S. C., BLOUT E. R. REVERSIBLE CONFORMATIONAL CHANGES OF MYOGLOBIN AND APOMYOGLOBIN. J Biol Chem. 1965 Jan;240:299–303. [PubMed] [Google Scholar]
- Hapner K. D., Bradshaw R. A., Hartzell C. R., Gurd F. R. Comparison of myoglobins from harbor seal, porpoise, and sperm whale. I. Preparation and characterization. J Biol Chem. 1968 Feb 25;243(4):683–689. [PubMed] [Google Scholar]
- Heber-Katz E., Hansburg D., Schwartz R. H. The Ia molecule of the antigen-presenting cell plays a critical role in immune response gene regulation of T cell activation. J Mol Cell Immunol. 1983;1(1):3–18. [PubMed] [Google Scholar]
- Hopgood M. F., Clark M. G., Ballard F. J. Inhibition of protein degradation in isolated rat hepatocytes. Biochem J. 1977 May 15;164(2):399–407. doi: 10.1042/bj1640399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishizaka K., Kishimoto T., Delespesse G., King T. P. Immunogenic properties of modified antigen E. I. Presence of specific determinants for T cells in denatured antigen and polypeptide chains. J Immunol. 1974 Jul;113(1):70–77. [PubMed] [Google Scholar]
- Jones W. C., Jr, Rothgeb T. M., Gurb F. R. Letter: Specific enrichment with 13-C of the methionine methyl groups of sperm whale myoglobin. J Am Chem Soc. 1975 Jun 25;97(13):3875–3877. doi: 10.1021/ja00846a085. [DOI] [PubMed] [Google Scholar]
- Jones W. C., Rothgeb T. M., Gurd F. R. Nuclear magnetic resonance studies of sperm whale myoglobin specifically enriched with 13C in the methionine methyl groups. J Biol Chem. 1976 Dec 10;251(23):7452–7460. [PubMed] [Google Scholar]
- Kimoto M., Fathman C. G. Antigen-reactive T cell clones. I. Transcomplementing hybrid I-A-region gene products function effectively in antigen presentation. J Exp Med. 1980 Oct 1;152(4):759–770. doi: 10.1084/jem.152.4.759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee K. C., Wong M., Spitzer D. Chloroquine as a probe for antigen processing by accessory cells. Transplantation. 1982 Sep;34(3):150–153. doi: 10.1097/00007890-198209000-00008. [DOI] [PubMed] [Google Scholar]
- Marshall R. C., Jones W. C., Jr, Vigna R. A., Gurd F. R. Isolation of cyanogen bromide cleavage peptides from myoglobins. Z Naturforsch C. 1974 Jan-Feb;29(1):90–91. [PubMed] [Google Scholar]
- Matis L. A., Longo D. L., Hedrick S. M., Hannum C., Margoliash E., Schwartz R. H. Clonal analysis of the major histocompatibility complex restriction and the fine specificity of antigen recognition in the T cell proliferative response to cytochrome C. J Immunol. 1983 Apr;130(4):1527–1535. [PubMed] [Google Scholar]
- Ohkuma S., Poole B. Fluorescence probe measurement of the intralysosomal pH in living cells and the perturbation of pH by various agents. Proc Natl Acad Sci U S A. 1978 Jul;75(7):3327–3331. doi: 10.1073/pnas.75.7.3327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenthal A. S. Determinant selection and macrophage function in genetic control of the immune response. Immunol Rev. 1978;40:136–152. doi: 10.1111/j.1600-065x.1978.tb00404.x. [DOI] [PubMed] [Google Scholar]
- Scala G., Oppenheim J. J. Antigen presentation by human monocytes: evidence for stimulant processing and requirement for interleukin 1. J Immunol. 1983 Sep;131(3):1160–1166. [PubMed] [Google Scholar]
- Schirrmacher V., Wigzell H. Immune responses against native and chemically modified albumins in mice. II. Effect of electric charge and conformation on the humoral antibody response and on helper T cell responses. J Immunol. 1974 Nov;113(5):1635–1643. [PubMed] [Google Scholar]
- Shimonkevitz R., Kappler J., Marrack P., Grey H. Antigen recognition by H-2-restricted T cells. I. Cell-free antigen processing. J Exp Med. 1983 Aug 1;158(2):303–316. doi: 10.1084/jem.158.2.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziegler H. K., Unanue E. R. Decrease in macrophage antigen catabolism caused by ammonia and chloroquine is associated with inhibition of antigen presentation to T cells. Proc Natl Acad Sci U S A. 1982 Jan;79(1):175–178. doi: 10.1073/pnas.79.1.175. [DOI] [PMC free article] [PubMed] [Google Scholar]


