Abstract
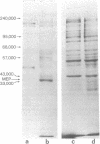
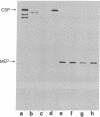
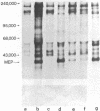
A protocol has been devised to radiolabel proteins secreted by murine fibroblasts in vitro. A radiolabeled polypeptide of molecular weight 35,000 is released into medium in relatively large amounts by transformed cells and in much smaller amounts by nontransformed fibroblasts. This major excreted polypeptide (MEP) is found in the medium of spontaneously transformed mouse cells and in the medium of mouse cells transformed by a DNA tumor virus, RNA tumor viruses, or methylcholanthrene. The appearance of MEP appears to be well correlated with anchorage independence in these transformed cells. MEP can be localized within the cytoplasm of transformed but not untransformed cells by indirect immunofluorescence. The presence of MEP within murine fibroblasts or in their culture medium serves as a novel biochemical marker of transformation. A biological role for this protein has not been assigned.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams S. L., Sobel M. E., Howard B. H., Olden K., Yamada K. M., de Crombrugghe B., Pastan I. Levels of translatable mRNAs for cell surface protein, collagen precursors, and two membrane proteins are altered in Rous sarcoma virus-transformed chick embryo fibroblasts. Proc Natl Acad Sci U S A. 1977 Aug;74(8):3399–3403. doi: 10.1073/pnas.74.8.3399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonner W. M., Laskey R. A. A film detection method for tritium-labelled proteins and nucleic acids in polyacrylamide gels. Eur J Biochem. 1974 Jul 1;46(1):83–88. doi: 10.1111/j.1432-1033.1974.tb03599.x. [DOI] [PubMed] [Google Scholar]
- Burke J. M., Balian G., Ross R., Bornstein P. Synthesis of types I and III procollagen and collagen by monkey aortic smooth muscle cells in vitro. Biochemistry. 1977 Jul 12;16(14):3243–3249. doi: 10.1021/bi00633a031. [DOI] [PubMed] [Google Scholar]
- Bürk R. R. A factor from a transformed cell line that affects cell migration. Proc Natl Acad Sci U S A. 1973 Feb;70(2):369–372. doi: 10.1073/pnas.70.2.369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen L. B., Buchanan J. M. Plasminogen-independent fibrinolysis by proteases produced by transformed chick embryo fibroblasts. Proc Natl Acad Sci U S A. 1975 Mar;72(3):1132–1136. doi: 10.1073/pnas.72.3.1132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Douglas M. G., Butow R. A. Variant forms of mitochondrial translation products in yeast: evidence for location of determinants on mitochondrial DNA. Proc Natl Acad Sci U S A. 1976 Apr;73(4):1083–1086. doi: 10.1073/pnas.73.4.1083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dulak N. C., Temin H. M. A partially purified polypeptide fraction from rat liver cell conditioned medium with multiplication-stimulating activity for embryo fibroblasts. J Cell Physiol. 1973 Apr;81(2):153–160. doi: 10.1002/jcp.1040810204. [DOI] [PubMed] [Google Scholar]
- Greenberger J. S., Aaronson S. A. Morphologic revertants of murine sarcoma virus transformed nonproducer BALB-3T3: selective techniques for isolation and biologic properties in vitro and in vivo. Virology. 1974 Feb;57(2):339–346. doi: 10.1016/0042-6822(74)90173-1. [DOI] [PubMed] [Google Scholar]
- Hammond M. E., Roblin R. O., Dvorak A. M., Selvaggio S. S., Black P. H., Dvorak H. F. MIF-like activity in simian virus 40-transformed 3T3 fibroblast cultures. Science. 1974 Sep 13;185(4155):955–957. doi: 10.1126/science.185.4155.955. [DOI] [PubMed] [Google Scholar]
- Hynes R. O. Alteration of cell-surface proteins by viral transformation and by proteolysis. Proc Natl Acad Sci U S A. 1973 Nov;70(11):3170–3174. doi: 10.1073/pnas.70.11.3170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klagsbrun M., Knighton D., Folkman J. Tumor angiogenesis activity in cells grown in tissue culture. Cancer Res. 1976 Jan;36(1):110–114. [PubMed] [Google Scholar]
- Laskey R. A., Mills A. D. Quantitative film detection of 3H and 14C in polyacrylamide gels by fluorography. Eur J Biochem. 1975 Aug 15;56(2):335–341. doi: 10.1111/j.1432-1033.1975.tb02238.x. [DOI] [PubMed] [Google Scholar]
- Levinson W., Bhatnagar R. S., Liu T. Z. Loss of ability to synthesize collagen in fibroblasts transformed by rous sarcoma virus. J Natl Cancer Inst. 1975 Oct;55(4):807–810. doi: 10.1093/jnci/55.4.807. [DOI] [PubMed] [Google Scholar]
- Olden K., Yamada K. M. Mechanism of the decrease in the major cell surface protein of chick embryo fibroblasts after transformation. Cell. 1977 Aug;11(4):957–969. doi: 10.1016/0092-8674(77)90307-5. [DOI] [PubMed] [Google Scholar]
- Ossowski L., Unkeless J. C., Tobia A., Quigley J. P., Rifkin D. B., Reich E. An enzymatic function associated with transformation of fibroblasts by oncogenic viruses. II. Mammalian fibroblast cultures transformed by DNA and RNA tumor viruses. J Exp Med. 1973 Jan 1;137(1):112–126. doi: 10.1084/jem.137.1.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterkofsky B. The effect of ascorbic acid on collagen polypeptide synthesis and proline hydroxylation during the growth of cultured fibroblasts. Arch Biochem Biophys. 1972 Sep;152(1):318–328. doi: 10.1016/0003-9861(72)90221-4. [DOI] [PubMed] [Google Scholar]
- Shin S. I., Freedman V. H., Risser R., Pollack R. Tumorigenicity of virus-transformed cells in nude mice is correlated specifically with anchorage independent growth in vitro. Proc Natl Acad Sci U S A. 1975 Nov;72(11):4435–4439. doi: 10.1073/pnas.72.11.4435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoker M., O'Neill C., Berryman S., Waxman V. Anchorage and growth regulation in normal and virus-transformed cells. Int J Cancer. 1968 Sep 15;3(5):683–693. doi: 10.1002/ijc.2910030517. [DOI] [PubMed] [Google Scholar]
- Studier F. W. Analysis of bacteriophage T7 early RNAs and proteins on slab gels. J Mol Biol. 1973 Sep 15;79(2):237–248. doi: 10.1016/0022-2836(73)90003-x. [DOI] [PubMed] [Google Scholar]
- Yamada K. M., Weston J. A. Isolation of a major cell surface glycoprotein from fibroblasts. Proc Natl Acad Sci U S A. 1974 Sep;71(9):3492–3496. doi: 10.1073/pnas.71.9.3492. [DOI] [PMC free article] [PubMed] [Google Scholar]