Abstract
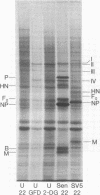
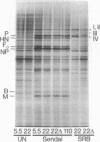
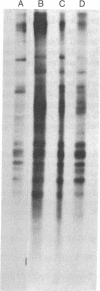
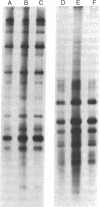
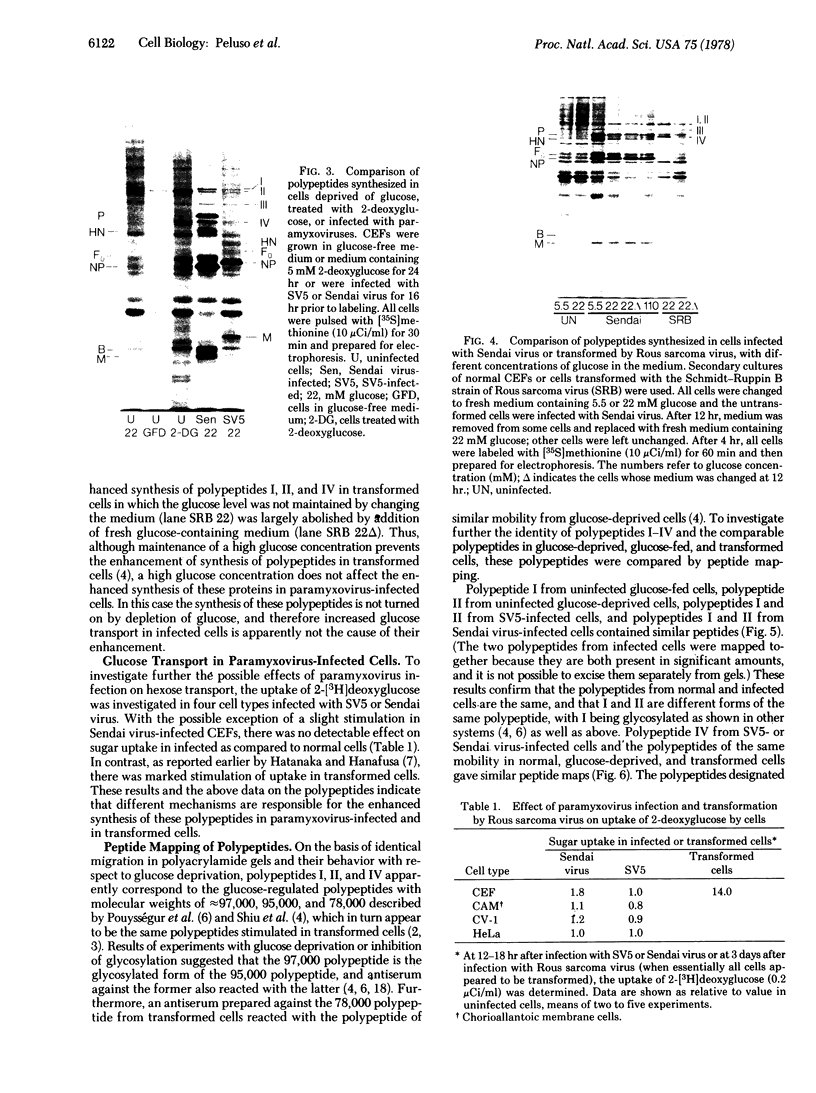
Infection of several types of cultured cells with the paramyxoviruses simian virus 5 and Sendai virus stimulates synthesis of four polypeptides (I-IV) with molecular weights of approximately 99,000, 97,000, 86,000, and 78,000, respectively. That these are host polypeptides encoded in cellular mRNAs has been shown by the inhibition of their synthesis by actinomycin D and by the similarity of the peptide maps of them and of polypeptides with the same electrophoretic mobility from uninfected cells. Peptide mapping as well as identical migration in polyacrylamide gels has also indicated that polypeptides I, II, and IV are the same as plasma membrane polypeptides whose synthesis is enhanced in cells transformed by Rous sarcoma virus and in normal cells by glucose deprivation or treatment with 2-deoxyglucose. Polypeptides I and II appear to be the same polypeptides, with the observed differences in migration reflecting the glycosylation of polypeptide I, a relationship previously shown to exist between polypeptides in glucose-deprived and glucose-fed cells. Infection with paramyxoviruses does not significantly increase the transport of glucose by cells, and the maintenance of a high concentration of glucose in the medium does not prevent the enhanced synthesis of these polypeptides. This is in contrast to the situation in transformed cells in which stimulation of synthesis of these polypeptides is secondary to depletion of glucose in the medium due to increased glucose uptake by the cells. Thus, although paramyxovirus infection and transformation by Rous sarcoma virus result in stimulation of the synthesis of the same membrane polypeptides, the mechanism of stimulation differs.
Keywords: simian virus 5, Sendai virus, membrane proteins, glucose uptake, glycosylation
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams S. L., Sobel M. E., Howard B. H., Olden K., Yamada K. M., de Crombrugghe B., Pastan I. Levels of translatable mRNAs for cell surface protein, collagen precursors, and two membrane proteins are altered in Rous sarcoma virus-transformed chick embryo fibroblasts. Proc Natl Acad Sci U S A. 1977 Aug;74(8):3399–3403. doi: 10.1073/pnas.74.8.3399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borland R., Mahy B. W. Deoxyribonucleic acid-dependent ribonucleic acid polymerase activity in cells infected with influenza virus. J Virol. 1968 Jan;2(1):33–39. doi: 10.1128/jvi.2.1.33-39.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choppin P. W. Effect of actinomycin D and halogenated deoxyuridines on the replication of simian virus 5. Proc Soc Exp Biol Med. 1965 Dec;120(3):699–702. doi: 10.3181/00379727-120-30629. [DOI] [PubMed] [Google Scholar]
- Cleveland D. W., Fischer S. G., Kirschner M. W., Laemmli U. K. Peptide mapping by limited proteolysis in sodium dodecyl sulfate and analysis by gel electrophoresis. J Biol Chem. 1977 Feb 10;252(3):1102–1106. [PubMed] [Google Scholar]
- Cursiefen D., Becht H. In vitro cultivation of cells from the chorioallantoic membrane of chick embryos. Med Microbiol Immunol. 1975;161(1):3–10. doi: 10.1007/BF02120764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg A. R. Increased protease levels in transformed cells: a casein overlay assay for the detection of plasminogen activator production. Cell. 1974 Jun;2(2):95–102. doi: 10.1016/0092-8674(74)90097-x. [DOI] [PubMed] [Google Scholar]
- Hatanaka M., Hanafusa H. Analysis of a functional change in membrane in the process of cell transformation by Rous sarcoma virus; alteration in the characteristics of sugar transport. Virology. 1970 Aug;41(4):647–652. doi: 10.1016/0042-6822(70)90429-0. [DOI] [PubMed] [Google Scholar]
- Holmes K. V., Choppin P. W. On the role of the response of the cell membrane in determining virus virulence. Contrasting effects of the parainfluenza virus SV5 in two cell types. J Exp Med. 1966 Sep 1;124(3):501–520. doi: 10.1084/jem.124.3.501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lamb R. A., Choppin P. W. Determination by peptide mapping of the unique polypeptides in Sendai virions and infected cells. Virology. 1978 Feb;84(2):469–478. doi: 10.1016/0042-6822(78)90263-5. [DOI] [PubMed] [Google Scholar]
- Lamb R. A., Choppin P. W. The synthesis of Sendai virus polypeptides in infected cells. III. Phosphorylation of polypeptides. Virology. 1977 Sep;81(2):382–397. doi: 10.1016/0042-6822(77)90154-4. [DOI] [PubMed] [Google Scholar]
- Lamb R. A., Etkind P. R., Choppin P. W. Evidence for a ninth influenza viral polypeptide. Virology. 1978 Nov;91(1):60–78. doi: 10.1016/0042-6822(78)90355-0. [DOI] [PubMed] [Google Scholar]
- Lamb R. A., Mahy B. W., Choppin P. W. The synthesis of sendai virus polypeptides in infected cells. Virology. 1976 Jan;69(1):116–131. doi: 10.1016/0042-6822(76)90199-9. [DOI] [PubMed] [Google Scholar]
- Lee S. G., Lipmann F. Isolation from normal and Rous sarcoma virus-transformed chicken fibroblasts of a factor that binds glucose and stimulates its transport. Proc Natl Acad Sci U S A. 1977 Jan;74(1):163–167. doi: 10.1073/pnas.74.1.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peluso R. W., Lamb R. A., Choppin P. W. Polypeptide synthesis in simian virus 5-infected cells. J Virol. 1977 Jul;23(1):177–187. doi: 10.1128/jvi.23.1.177-187.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pouysségur J., Shiu R. P., Pastan I. Induction of two transformation-sensitive membrane polypeptides in normal fibroblasts by a block in glycoprotein synthesis or glucose deprivation. Cell. 1977 Aug;11(4):941–947. doi: 10.1016/0092-8674(77)90305-1. [DOI] [PubMed] [Google Scholar]
- Pouysségur J., Yamada K. M. Isolation and immunological characterization of a glucose-regulated fibroblast cell surface glycoprotein and its nonglycosylated precursor. Cell. 1978 Jan;13(1):139–140. doi: 10.1016/0092-8674(78)90145-9. [DOI] [PubMed] [Google Scholar]
- Shiu R. P., Pouyssegur J., Pastan I. Glucose depletion accounts for the induction of two transformation-sensitive membrane proteinsin Rous sarcoma virus-transformed chick embryo fibroblasts. Proc Natl Acad Sci U S A. 1977 Sep;74(9):3840–3844. doi: 10.1073/pnas.74.9.3840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stone K. R., Smith R. E., Joklik W. K. Changes in membrane polypeptides that occur when chick embryo fibroblasts and NRK cells are transformed with avian sarcoma viruses. Virology. 1974 Mar;58(1):86–100. doi: 10.1016/0042-6822(74)90143-3. [DOI] [PubMed] [Google Scholar]