Abstract
The analysis of H-2K products from spontaneously generated major histocompatibility complex (MHC) mutants and of the primary structure of other class I antigens suggests the genetic hypothesis that diversity in the MHC results from a copy mechanism analogous to gene conversion. The hypothesis was tested by making precise structural predictions about three partially characterized MHC mutants (bm1, bm3, and bm8). The predictions were based on consensus sequences among class I genes that differ from H-2Kb in the same region of the molecule as do the Kb mutants. In two cases (bm3 and bm8) we successfully predicted the correct amino acid substitution at positions known to be altered but for which the specific nature of the substitution had not been determined. In two additional cases (bm1 and bm8) we predicted and found both new mutation sites and the specific amino acid substitutions. The positions and identifications of the variant amino acids were determined by radiolabeled amino acid sequence analysis and DNA restriction endonuclease analysis. The interaction of MHC genes through a copy mechanism to generate diversity permits the introduction of multiple nucleotide base substitutions into class I sequences by a single genetic event. Such a mechanism may account in part for the large structural divergence among alleles of MHC loci and the high degree of MHC polymorphism among wild mice.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baltimore D. Gene conversion: some implications for immunoglobulin genes. Cell. 1981 Jun;24(3):592–594. doi: 10.1016/0092-8674(81)90082-9. [DOI] [PubMed] [Google Scholar]
- Bodmer W. F. New genetic model for allelism at histocompatibility and other complex loci: polymorphism for control of gene expression. Transplant Proc. 1973 Dec;5(4):1471–1475. [PubMed] [Google Scholar]
- Brown J. L., Kato K., Silver J., Nathenson S. G. Notable diversity in peptide composition of murine H-2K and H-2D alloantigens. Biochemistry. 1974 Jul 16;13(15):3174–3178. doi: 10.1021/bi00712a027. [DOI] [PubMed] [Google Scholar]
- Brown J. L., Nathenson S. G. Structural differences between parent and mutant H-2K glycoproteins from two H-2K gene mutants: b6.c-h-2ba (Hzl) and B6-H-2bd (M505). J Immunol. 1977 Jan;118(1):98–102. [PubMed] [Google Scholar]
- Clarke S. H., Claflin J. L., Rudikoff S. Polymorphism in immunoglobulin heavy chains suggesting gene conversion. Proc Natl Acad Sci U S A. 1982 May;79(10):3280–3284. doi: 10.1073/pnas.79.10.3280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coligan J. E., Kindt T. J., Nairn R., Nathenson S. G., Sachs D. H., Hansen T. H. Primary structural studies of an H-2L molecule confirm that it is a unique gene product with homology to H-2K and H-2D antigens. Proc Natl Acad Sci U S A. 1980 Feb;77(2):1134–1138. doi: 10.1073/pnas.77.2.1134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egel R. Intergenic conversion and reiterated genes. Nature. 1981 Mar 19;290(5803):191–192. doi: 10.1038/290191a0. [DOI] [PubMed] [Google Scholar]
- Evans G. A., Margulies D. H., Camerini-Otero R. D., Ozato K., Seidman J. G. Structure and expression of a mouse major histocompatibility antigen gene, H-2Ld. Proc Natl Acad Sci U S A. 1982 Mar;79(6):1994–1998. doi: 10.1073/pnas.79.6.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ewenstein B. M., Uehara H., Nisizawa T., Melvold R. W., Kohn H. I., Nathenson S. G. Biochemical studies on the H-2K antigens of the MHC mutants bm3 and bm11. Immunogenetics. 1980;11(4):383–395. doi: 10.1007/BF01567805. [DOI] [PubMed] [Google Scholar]
- Günther E., Stark O. Overview. The major histocompatibility system of the rat. Transplant Proc. 1979 Sep;11(3):1550–1553. [PubMed] [Google Scholar]
- Hozumi N., Tonegawa S. Evidence for somatic rearrangement of immunoglobulin genes coding for variable and constant regions. Proc Natl Acad Sci U S A. 1976 Oct;73(10):3628–3632. doi: 10.1073/pnas.73.10.3628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang C. M., Parsons M., Wakeland E. K., Moriwaki K., Herzenberg L. A. New immunoglobulin IgG allotypes and haplotypes found in wild mice with monoclonal anti-allotope antibodies. J Immunol. 1982 Feb;128(2):661–667. [PubMed] [Google Scholar]
- Johnson R. E., Wells M. A., Rupley J. A. Thermodynamics of dihexanoylphosphatidylcholine aggregation. Biochemistry. 1981 Jul 7;20(14):4239–4242. doi: 10.1021/bi00517a044. [DOI] [PubMed] [Google Scholar]
- Kimball E. S., Nathenson S. G., Coligan J. E. Amino acid sequence of residues 1-98 of the K-2Kb murine major histocompatibility alloantigen: comparison with H-2Kb and H-2db reveals extensive localized differences. Biochemistry. 1981 May 26;20(11):3301–3308. doi: 10.1021/bi00514a049. [DOI] [PubMed] [Google Scholar]
- Klein J., Figueroa F. Polymorphism of the mouse H-2 loci. Immunol Rev. 1981;60:23–57. doi: 10.1111/j.1600-065x.1981.tb00361.x. [DOI] [PubMed] [Google Scholar]
- Lehmann H., Carrell R. W. Variations in the structure of human haemoglobin. With particular reference to the unstable haemoglobins. Br Med Bull. 1969 Jan;25(1):14–23. doi: 10.1093/oxfordjournals.bmb.a070664. [DOI] [PubMed] [Google Scholar]
- López de Castro J. A., Strominger J. L., Strong D. M., Orr H. T. Structure of crossreactive human histocompatibility antigens HLA-A28 and HLA-A2: possible implications for the generation of HLA polymorphism. Proc Natl Acad Sci U S A. 1982 Jun;79(12):3813–3817. doi: 10.1073/pnas.79.12.3813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maloy W. L., Coligan J. E. Primary structure of the H-2Db alloantigen. II. Additional amino acid sequence information, localization of a third site of glycosylation and evidence for K and D region specific sequences. Immunogenetics. 1982;16(1):11–22. doi: 10.1007/BF00364438. [DOI] [PubMed] [Google Scholar]
- Melvold R. W., Kohn H. I., Dunn G. R. History and genealogy of the H-2Kb mutants from the C57BL/6Kh colony. Immunogenetics. 1982;15(2):177–185. doi: 10.1007/BF00621950. [DOI] [PubMed] [Google Scholar]
- Moore K. W., Sher B. T., Sun Y. H., Eakle K. A., Hood L. DNA sequence of a gene encoding a BALB/c mouse Ld transplantation antigen. Science. 1982 Feb 5;215(4533):679–682. doi: 10.1126/science.7058332. [DOI] [PubMed] [Google Scholar]
- Nairn R., Yamaga K., Nathenson S. G. Biochemistry of the gene products from murine MHC mutants. Annu Rev Genet. 1980;14:241–277. doi: 10.1146/annurev.ge.14.120180.001325. [DOI] [PubMed] [Google Scholar]
- Nathenson S. G., Uehara H., Ewenstein B. M., Kindt T. J., Coligan J. E. Primary structural: analysis of the transplantation antigens of the murine H-2 major histocompatibility complex. Annu Rev Biochem. 1981;50:1025–1052. doi: 10.1146/annurev.bi.50.070181.005113. [DOI] [PubMed] [Google Scholar]
- Nguyen H. T., Gubits R. M., Wydro R. M., Nadal-Ginard B. Sarcomeric myosin heavy chain is coded by a highly conserved multigene family. Proc Natl Acad Sci U S A. 1982 Sep;79(17):5230–5234. doi: 10.1073/pnas.79.17.5230. [DOI] [PMC free article] [PubMed] [Google Scholar]
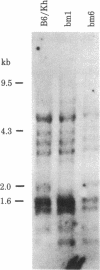
- Nisizawa T., Ewenstein B. M., Uehara H., McGovern D., Nathenson S. G. Biochemical studies on the H-2K antigens of the MHC mutant bml. Immunogenetics. 1981;12(1-2):33–44. doi: 10.1007/BF01561649. [DOI] [PubMed] [Google Scholar]
- Pease L. R., Nathenson S. G., Leinwand L. A. Mapping class I gene sequences in the major histocompatibility complex. Nature. 1982 Jul 22;298(5872):382–385. doi: 10.1038/298382a0. [DOI] [PubMed] [Google Scholar]
- Reyes A. A., Schöld M., Itakura K., Wallace R. B. Isolation of a cDNA clone for the murine transplantation antigen H-2Kb. Proc Natl Acad Sci U S A. 1982 May;79(10):3270–3274. doi: 10.1073/pnas.79.10.3270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reyes A. A., Schöld M., Wallace R. B. The complete amino acid sequence of the murine transplantation antigen H-2Db as deduced by molecular cloning. Immunogenetics. 1982;16(1):1–9. doi: 10.1007/BF00364437. [DOI] [PubMed] [Google Scholar]
- Rothbard J. B., Hopp T. P., Edelman G. M., Cunningham B. A. Structure of the heavy chain of the H-2Kk histocompatibility antigen. Proc Natl Acad Sci U S A. 1980 Jul;77(7):4239–4243. doi: 10.1073/pnas.77.7.4239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silver J., Hood L. Preliminary amino acid sequences of transplantation antigens: genetic and evolutionary implications. Contemp Top Mol Immunol. 1976;5:35–68. doi: 10.1007/978-1-4684-8142-6_2. [DOI] [PubMed] [Google Scholar]
- Slightom J. L., Blechl A. E., Smithies O. Human fetal G gamma- and A gamma-globin genes: complete nucleotide sequences suggest that DNA can be exchanged between these duplicated genes. Cell. 1980 Oct;21(3):627–638. doi: 10.1016/0092-8674(80)90426-2. [DOI] [PubMed] [Google Scholar]
- Steinmetz M., Frelinger J. G., Fisher D., Hunkapiller T., Pereira D., Weissman S. M., Uehara H., Nathenson S., Hood L. Three cDNA clones encoding mouse transplantation antigens: homology to immunoglobulin genes. Cell. 1981 Apr;24(1):125–134. doi: 10.1016/0092-8674(81)90508-0. [DOI] [PubMed] [Google Scholar]
- Steinmetz M., Moore K. W., Frelinger J. G., Sher B. T., Shen F. W., Boyse E. A., Hood L. A pseudogene homologous to mouse transplantation antigens: transplantation antigens are encoded by eight exons that correlate with protein domains. Cell. 1981 Sep;25(3):683–692. doi: 10.1016/0092-8674(81)90175-6. [DOI] [PubMed] [Google Scholar]
- Uehara H., Ewenstein B. M., Martinko J. M., Nathenson S. G., Kindt T. J., Coligan J. E. Primary structure of murine major histocompatibility alloantigens: amino acid sequence of the cyanogen bromide fragment Ia (positions 139-228) from the H-2Kb molecule. Biochemistry. 1980 Dec 23;19(26):6182–6188. doi: 10.1021/bi00567a036. [DOI] [PubMed] [Google Scholar]