Abstract
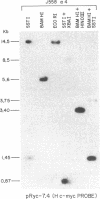
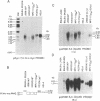
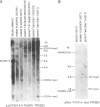
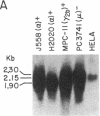
NIARD (non-immunoglobulin-associated rearranging DNA) is located on mouse chromosome 15 at the break point of a commonly observed translocation event involving chromosomes 15 and 12 in murine plasmacytomas. The human cellular analogue of the v-myc oncogene of avian myelocytomatosis virus, strain MC-29, is known to reside on the distal end of human chromosome 8 and has been observed to translocate to chromosome 14 in Burkitt lymphomas. Using a cDNA clone specific for the transcript of the human c-myc gene (H c-myc), we show that the mouse c-myc (M c-myc) gene is contained within NIARD. NIARD-associated chromosome translocations occurred 1.3-2 kilobases (kb) 5′ of the mouse c-myc gene where NIARD recombines with the switch region of the Cα immunoglobulin gene in various murine plasmacytomas. The mouse c-myc encoding region within NIARD spanned <2.4 kb of DNA and expressed a low level of a 2.3-kb polyadenylylated RNA in BALB/c spleen. Increased (10- to 20-fold) levels of rearranged mouse c-myc transcripts (i.e., ≈1.8-2.1 kb) were observed in plasmacytomas that have NIARD-associated chromosome translocations. Human c-myc and NIARD probes detected DNA rearrangements of human c-myc in four of seven Burkitt lymphomas. DNA sequences adjacent to the human c-myc gene recombined with the Cμ immunoglobulin gene locus on chromosome 14 in several Burkitt lymphomas. The activation of the c-myc oncogene by chromosome translocation implicates its involvement in B-cell oncogenesis.
Keywords: NIARD (non-immunoglobulin-associated rearranging DNA), transcription activation by chromosome translocation
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams J. M., Gerondakis S., Webb E., Mitchell J., Bernard O., Cory S. Transcriptionally active DNA region that rearranges frequently in murine lymphoid tumors. Proc Natl Acad Sci U S A. 1982 Nov;79(22):6966–6970. doi: 10.1073/pnas.79.22.6966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benjamin D., Magrath I. T., Maguire R., Janus C., Todd H. D., Parsons R. G. Immunoglobulin secretion by cell lines derived from African and American undifferentiated lymphomas of Burkitt's and non-Burkitt's type. J Immunol. 1982 Sep;129(3):1336–1342. [PubMed] [Google Scholar]
- Calame K., Kim S., Lalley P., Hill R., Davis M., Hood L. Molecular cloning of translocations involving chromosome 15 and the immunoglobulin C alpha gene from chromosome 12 in two murine plasmacytomas. Proc Natl Acad Sci U S A. 1982 Nov;79(22):6994–6998. doi: 10.1073/pnas.79.22.6994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Cooper G. M. Cellular transforming genes. Science. 1982 Aug 27;217(4562):801–806. doi: 10.1126/science.6285471. [DOI] [PubMed] [Google Scholar]
- Croce C. M., Shander M., Martinis J., Cicurel L., D'Ancona G. G., Dolby T. W., Koprowski H. Chromosomal location of the genes for human immunoglobulin heavy chains. Proc Natl Acad Sci U S A. 1979 Jul;76(7):3416–3419. doi: 10.1073/pnas.76.7.3416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalla-Favera R., Bregni M., Erikson J., Patterson D., Gallo R. C., Croce C. M. Human c-myc onc gene is located on the region of chromosome 8 that is translocated in Burkitt lymphoma cells. Proc Natl Acad Sci U S A. 1982 Dec;79(24):7824–7827. doi: 10.1073/pnas.79.24.7824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalla-Favera R., Gelmann E. P., Martinotti S., Franchini G., Papas T. S., Gallo R. C., Wong-Staal F. Cloning and characterization of different human sequences related to the onc gene (v-myc) of avian myelocytomatosis virus (MC29). Proc Natl Acad Sci U S A. 1982 Nov;79(21):6497–6501. doi: 10.1073/pnas.79.21.6497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erikson J., Finan J., Nowell P. C., Croce C. M. Translocation of immunoglobulin VH genes in Burkitt lymphoma. Proc Natl Acad Sci U S A. 1982 Sep;79(18):5611–5615. doi: 10.1073/pnas.79.18.5611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldfarb M., Shimizu K., Perucho M., Wigler M. Isolation and preliminary characterization of a human transforming gene from T24 bladder carcinoma cells. Nature. 1982 Apr 1;296(5856):404–409. doi: 10.1038/296404a0. [DOI] [PubMed] [Google Scholar]
- Harris L. J., D'Eustachio P., Ruddle F. H., Marcu K. B. DNA sequence associated with chromosome translocations in mouse plasmacytomas. Proc Natl Acad Sci U S A. 1982 Nov;79(21):6622–6626. doi: 10.1073/pnas.79.21.6622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris L. J., Lang R. B., Marcu K. B. Non-immunoglobulin-associated DNA rearrangements in mouse plasmacytomas. Proc Natl Acad Sci U S A. 1982 Jul;79(13):4175–4179. doi: 10.1073/pnas.79.13.4175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayward W. S., Neel B. G., Astrin S. M. Activation of a cellular onc gene by promoter insertion in ALV-induced lymphoid leukosis. Nature. 1981 Apr 9;290(5806):475–480. doi: 10.1038/290475a0. [DOI] [PubMed] [Google Scholar]
- Hu N., Messing J. The making of strand-specific M13 probes. Gene. 1982 Mar;17(3):271–277. doi: 10.1016/0378-1119(82)90143-3. [DOI] [PubMed] [Google Scholar]
- Kirsch I. R., Morton C. C., Nakahara K., Leder P. Human immunoglobulin heavy chain genes map to a region of translocations in malignant B lymphocytes. Science. 1982 Apr 16;216(4543):301–303. doi: 10.1126/science.6801764. [DOI] [PubMed] [Google Scholar]
- Klein G. The role of gene dosage and genetic transpositions in carcinogenesis. Nature. 1981 Nov 26;294(5839):313–318. doi: 10.1038/294313a0. [DOI] [PubMed] [Google Scholar]
- Lautenberger J. A., Schulz R. A., Garon C. F., Tsichlis P. N., Papas T. S. Molecular cloning of avian myelocytomatosis virus (MC29) transforming sequences. Proc Natl Acad Sci U S A. 1981 Mar;78(3):1518–1522. doi: 10.1073/pnas.78.3.1518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manolov G., Manolova Y. Marker band in one chromosome 14 from Burkitt lymphomas. Nature. 1972 May 5;237(5349):33–34. doi: 10.1038/237033a0. [DOI] [PubMed] [Google Scholar]
- Marcu K. B., Valbuena O., Perry R. P. Isolation, purification, and properties of mouse heavy-chain immunoglobulin mRNAs. Biochemistry. 1978 May 2;17(9):1723–1733. doi: 10.1021/bi00602a022. [DOI] [PubMed] [Google Scholar]
- Neel B. G., Hayward W. S., Robinson H. L., Fang J., Astrin S. M. Avian leukosis virus-induced tumors have common proviral integration sites and synthesize discrete new RNAs: oncogenesis by promoter insertion. Cell. 1981 Feb;23(2):323–334. doi: 10.1016/0092-8674(81)90128-8. [DOI] [PubMed] [Google Scholar]
- Ohno S., Babonits M., Wiener F., Spira J., Klein G., Potter M. Nonrandom chromosome changes involving the Ig gene-carrying chromosomes 12 and 6 in pristane-induced mouse plasmacytomas. Cell. 1979 Dec;18(4):1001–1007. doi: 10.1016/0092-8674(79)90212-5. [DOI] [PubMed] [Google Scholar]
- Payne G. S., Courtneidge S. A., Crittenden L. B., Fadly A. M., Bishop J. M., Varmus H. E. Analysis of avian leukosis virus DNA and RNA in bursal tumours: viral gene expression is not required for maintenance of the tumor state. Cell. 1981 Feb;23(2):311–322. doi: 10.1016/0092-8674(81)90127-6. [DOI] [PubMed] [Google Scholar]
- Schibler U., Marcu K. B., Perry R. P. The synthesis and processing of the messenger RNAs specifying heavy and light chain immunoglobulins in MPC-11 cells. Cell. 1978 Dec;15(4):1495–1509. doi: 10.1016/0092-8674(78)90072-7. [DOI] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Thomas P. S. Hybridization of denatured RNA and small DNA fragments transferred to nitrocellulose. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5201–5205. doi: 10.1073/pnas.77.9.5201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiener F., Babonits M., Spira J., Klein G., Potter M. Cytogenetic studies on IgA/lambda-producing murine plasmacytomas: regular occurrence of a T(12;15) translocation. Somatic Cell Genet. 1980 Nov;6(6):731–738. doi: 10.1007/BF01538972. [DOI] [PubMed] [Google Scholar]
- Zech L., Haglund U., Nilsson K., Klein G. Characteristic chromosomal abnormalities in biopsies and lymphoid-cell lines from patients with Burkitt and non-Burkitt lymphomas. Int J Cancer. 1976 Jan 15;17(1):47–56. doi: 10.1002/ijc.2910170108. [DOI] [PubMed] [Google Scholar]