Abstract
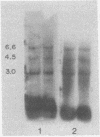
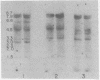
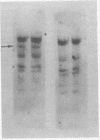
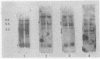
Human cellular DNA fragments from cells of normal subjects and patients with thalassemia obtained by restriction enzyme digestion were analyzed for their globin gene content. The fragments were separated on agarose gels, transferred to nitrocellulose filters, hybridized to globin [32P]cDNA, and radioautographed. One to ten picograms of globin gene sequences were detectable. With EcoRI digestion, eight to nine cellular DNA fragments were found to contain globin genes. Three of these contained β-like gene sequences assayed with β globin cDNA probe. One β-like fragment was absent in DNA from a homozygous subject for hemoglobin Lepore. Two of the three β gene-containing fragments present in normal DNA were absent in DNA from a patient with hereditary persistence of fetal hemoglobin. The same two fragments containing β-like genes were absent from δβ thalassemic DNA and one new fragment containing β-like genes was found. Together with results obtained by hybridization of these DNAs in solution, the data are consistent with deletion of specific restriction human DNA fragments in subjects with these disorders and a greater deletion of β-like gene sequences in subjects with hereditary persistence of fetal hemoglobin than in those with δβ thalassemia.
Keywords: β thalassemia, restriction enzyme digestion, “blot” hybridization, cellular DNA
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BAGLIONI C. The fusion of two peptide chains in hemoglobin Lepore and its interpretation as a genetic deletion. Proc Natl Acad Sci U S A. 1962 Nov 15;48:1880–1886. doi: 10.1073/pnas.48.11.1880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Botchan M., Topp W., Sambrook J. The arrangement of simian virus 40 sequences in the DNA of transformed cells. Cell. 1976 Oct;9(2):269–287. doi: 10.1016/0092-8674(76)90118-5. [DOI] [PubMed] [Google Scholar]
- Britten R. J., Kohne D. E. Repeated sequences in DNA. Hundreds of thousands of copies of DNA sequences have been incorporated into the genomes of higher organisms. Science. 1968 Aug 9;161(3841):529–540. doi: 10.1126/science.161.3841.529. [DOI] [PubMed] [Google Scholar]
- Cann A., Gambino R., Banks J., Bank A. Polyadenylate sequences and biologic activity of human globin messenger ribonucleic acid. J Biol Chem. 1974 Dec 10;249(23):7536–7540. [PubMed] [Google Scholar]
- Duesberg P. H., Vogt P. K. Gel electrophoresis of avian leukosis and sarcoma viral RNA in formamide: comparison with other viral and cellular RNA species. J Virol. 1973 Sep;12(3):594–599. doi: 10.1128/jvi.12.3.594-599.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forget B. G., Hillman D. G., Lazarus H., Barell E. F., Benz ej J. R., Caskey C. T., Huisman T. H., Schroeder W. A., Housman D. Absence of messenger RNA and gene DNA for beta-globin chains in hereditary persistence of fetal hemoglobin. Cell. 1976 Mar;7(3):323–329. doi: 10.1016/0092-8674(76)90161-6. [DOI] [PubMed] [Google Scholar]
- Gambino R., Kacian D., O'Donnell J., Ramirez F., Marks P. A., Bank A. A limited number of globin genes in human DNA. Proc Natl Acad Sci U S A. 1974 Oct;71(10):3966–3970. doi: 10.1073/pnas.71.10.3966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeffreys A. J., Flavell R. A. A physical map of the DNA regions flanking the rabbit beta-globin gene. Cell. 1977 Oct;12(2):429–439. doi: 10.1016/0092-8674(77)90119-2. [DOI] [PubMed] [Google Scholar]
- Jeffreys A. J., Flavell R. A. The rabbit beta-globin gene contains a large large insert in the coding sequence. Cell. 1977 Dec;12(4):1097–1108. doi: 10.1016/0092-8674(77)90172-6. [DOI] [PubMed] [Google Scholar]
- Kacian D. L., Myers J. C. Synthesis of extensive, possibly complete, DNA copies of poliovirus RNA in high yields and at high specific activities. Proc Natl Acad Sci U S A. 1976 Jul;73(7):2191–2195. doi: 10.1073/pnas.73.7.2191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kan Y. W., Holland J. P., Dozy A. M., Charache S., Kazazian H. H. Deletion of the beta-globin structure gene in hereditary persistence of foetal haemoglobin. Nature. 1975 Nov 13;258(5531):162–163. doi: 10.1038/258162a0. [DOI] [PubMed] [Google Scholar]
- Ketner G., Kelly T. J., Jr Integrated simian virus 40 sequences in transformed cell DNA: analysis using restriction endonucleases. Proc Natl Acad Sci U S A. 1976 Apr;73(4):1102–1106. doi: 10.1073/pnas.73.4.1102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marotta C. A., Wilson J. T., Forget B. G., Weissman S. M. Human beta-globin messenger RNA. III. Nucleotide sequences derived from complementary DNA. J Biol Chem. 1977 Jul 25;252(14):5040–5053. [PubMed] [Google Scholar]
- McDonell M. W., Simon M. N., Studier F. W. Analysis of restriction fragments of T7 DNA and determination of molecular weights by electrophoresis in neutral and alkaline gels. J Mol Biol. 1977 Feb 15;110(1):119–146. doi: 10.1016/s0022-2836(77)80102-2. [DOI] [PubMed] [Google Scholar]
- Nudel U., Ramirez F., Marks P. A., Bank A. Preparative polyacrylamide gel electrophoretic purification of human alpha- and beta-globin messenger RNAs. J Biol Chem. 1977 Apr 10;252(7):2182–2186. [PubMed] [Google Scholar]
- Ottolenghi S., Comi P., Giglioni B., Tolstoshev P., Lanyon W. G., Mitchell G. J., Williamson R., Russo G., Musumeci S., Schillro G. Delta-beta-thalassemia is due to a gene deletion. Cell. 1976 Sep;9(1):71–80. doi: 10.1016/0092-8674(76)90053-2. [DOI] [PubMed] [Google Scholar]
- Ottolenghi S., Lanyon W. G., Paul J., Williamson R., Weatherall D. J., Clegg J. B., Pritchard J., Pootrakul S., Boon W. H. The severe form of alpha thalassaemia is caused by a haemoglobin gene deletion. Nature. 1974 Oct 4;251(5474):389–392. doi: 10.1038/251389a0. [DOI] [PubMed] [Google Scholar]
- Ramirez F., Natta C., O'Donnell J. V., Canale V., Bailey G., Sanguensermsri T., Maniatis G. M., Marks P. A., Bank A. Relative numbers of human globin genes assayed with purified alpha and beta complementary human DNA. Proc Natl Acad Sci U S A. 1975 Apr;72(4):1550–1554. doi: 10.1073/pnas.72.4.1550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramirez F., O'Donnell J. V., Marks P. A., Bank A., Musumeci S., Schilirò G., Pizzarelli G., Russo G., Luppis B., Gambino R. Abnormal or absent beta mRNA in betao Ferrara and gene deletion in delta beta thalassaemia. Nature. 1976 Oct 7;263(5577):471–475. doi: 10.1038/263471a0. [DOI] [PubMed] [Google Scholar]
- Ringelhann B., Konotey-Ahulu F. I., Lehmann H., Lorkin P. A. A Ghanaian adult, homozygous for hereditary persistence of foetal haemoglobin and heterozygous for elliptocytosis. Acta Haematol. 1970;43(2):100–110. doi: 10.1159/000208719. [DOI] [PubMed] [Google Scholar]
- SMITHIES O. CHROMOSOMAL REARRANGEMENTS AND PROTEIN STRUCTURE. Cold Spring Harb Symp Quant Biol. 1964;29:309–319. doi: 10.1101/sqb.1964.029.01.033. [DOI] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Taylor J. M., Dozy A., Kan Y. W., Varmus H. E., Lie-Injo L. E., Ganesan J., Todd D. Genetic lesion in homozygous alpha thalassaemia (hydrops fetalis). Nature. 1974 Oct 4;251(5474):392–393. doi: 10.1038/251392a0. [DOI] [PubMed] [Google Scholar]
- Tilghman S. M., Tiemeier D. C., Seidman J. G., Peterlin B. M., Sullivan M., Maizel J. V., Leder P. Intervening sequence of DNA identified in the structural portion of a mouse beta-globin gene. Proc Natl Acad Sci U S A. 1978 Feb;75(2):725–729. doi: 10.1073/pnas.75.2.725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tolstoshev P., Mitchell J., Lanyon G., Williamson R., Ottolenghi S., Comi P., Giglioni B., Masera G., Modell B., Weatherall D. J. Presence of gene for beta globin in homozygous beta0 thalassaemia. Nature. 1976 Jan 15;259(5539):95–98. doi: 10.1038/259095a0. [DOI] [PubMed] [Google Scholar]
- Wilson J. T., deRiel J. K., Forget B. G., Marotta C. A., Weissman S. M. Nucleotide sequence of 3' untranslated portion of human alpha globin mRNA. Nucleic Acids Res. 1977 Jul;4(7):2353–2368. doi: 10.1093/nar/4.7.2353. [DOI] [PMC free article] [PubMed] [Google Scholar]