Abstract
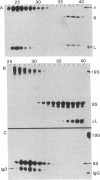
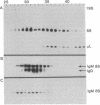
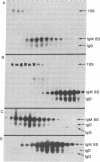
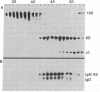
The gel filtration behavior, in the presence of detergents, of membrane-bound IgM from normal mouse spleen B lymphocytes was compared to that of secretory IgM from mouse plasma cells. The proteins were labeled either by surface radioiodination or biosynthetically with radioactive amino acids. Cell lysates were fractionated on calibrated Sepharose 6B columns in the presence of the detergents Nonidet P-40 or deoxycholate. Eluted fractions were immunoprecipitated and the reduced or unreduced precipitates were analyzed by sodium dodecyl sulfate gel electrophoresis followed by radioautography. Surface 125I-labeled 8S IgM exhibited a gel filtration pattern in Nonidet P-40 corresponding to much higher apparent molecular weight than that of secretory 8S IgM, a difference that almost disappeared when gel filtration was performed in the presence of deoxycholate, which forms much smaller micelles than does Nonidet P-40. Biosynthetically labeled lymphocytes contain two types of IgM molecules differing in their gel filtration behavior and fate: one identical to secretory 8S IgM of plasma cells and secreted in the medium during chase periods, and the other identical to surface 125I-labeled IgM and remaining cell-associated. Because the surface-bound 8S IgM was not found to be associated with other labeled molecules, it is likely that the detergent-binding behavior of surface IgM is due to a hydrophobic segment carried by these Ig molecules. That lymphocytes synthesize two types of μ chains was also shown by the use of tunicamycin, an inhibitor of glycosylation. In its presence, two unglycosylated μ chains were observed: one identical in size to that made by tunicamycin-treated plasma cells, and the second slightly larger. Gel filtration in Nonidet P-40 of the cell lysates of tunicamycin-treated lymphocytes showed that the nonsecretory 8S IgM contains this second type of μ chains, whereas the IgM molecules of the secretory type contain plasma cell-like μ chains. It is suggested that membrane IgM μ chains contain a hydrophobic segment which is responsible for its association to the membrane.
Keywords: membrane immunoglobulin, hydrophobicity, detergent binding, tunicamycin, glycosylation
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bergman Y., Haimovich J. B lymphocytes contain three species of mu chains. Eur J Immunol. 1978 Dec;8(12):876–880. doi: 10.1002/eji.1830081210. [DOI] [PubMed] [Google Scholar]
- Bonner W. M., Laskey R. A. A film detection method for tritium-labelled proteins and nucleic acids in polyacrylamide gels. Eur J Biochem. 1974 Jul 1;46(1):83–88. doi: 10.1111/j.1432-1033.1974.tb03599.x. [DOI] [PubMed] [Google Scholar]
- Davidson W. F., Parish C. R. A procedure for removing red cells and dead cells from lymphoid cell suspensions. J Immunol Methods. 1975 Jun;7(2-3):291–300. doi: 10.1016/0022-1759(75)90026-5. [DOI] [PubMed] [Google Scholar]
- Early P. W., Davis M. M., Kaback D. B., Davidson N., Hood L. Immunoglobulin heavy chain gene organization in mice: analysis of a myeloma genomic clone containing variable and alpha constant regions. Proc Natl Acad Sci U S A. 1979 Feb;76(2):857–861. doi: 10.1073/pnas.76.2.857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helenius A., Simons K. Solubilization of membranes by detergents. Biochim Biophys Acta. 1975 Mar 25;415(1):29–79. doi: 10.1016/0304-4157(75)90016-7. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- McIlhinney R. A., Richardson N. E., Feinstein A. Evidence for a C-terminal tyrosine residue in human and mouse B-lymphocyte membrane mu chains. Nature. 1978 Apr 6;272(5653):555–557. doi: 10.1038/272555a0. [DOI] [PubMed] [Google Scholar]
- Melcher U., Uhr J. W. Cell surface immunoglobulin. XVI. Polypeptide chain structure of mouse IgM and IgD-like molecule. J Immunol. 1976 Feb;116(2):409–415. [PubMed] [Google Scholar]
- Melcher U., Uhr J. W. Density differences between membrane and secreted immunoglobins of murine splenocytes. Biochemistry. 1977 Jan 11;16(1):145–152. doi: 10.1021/bi00620a025. [DOI] [PubMed] [Google Scholar]
- Ryser J. E., Vassalli P. Mouse bone marrow lymphocytes and their differentiation. J Immunol. 1974 Sep;113(3):719–728. [PubMed] [Google Scholar]
- Sakano H., Rogers J. H., Hüppi K., Brack C., Traunecker A., Maki R., Wall R., Tonegawa S. Domains and the hinge region of an immunoglobulin heavy chain are encoded in separate DNA segments. Nature. 1979 Feb 22;277(5698):627–633. doi: 10.1038/277627a0. [DOI] [PubMed] [Google Scholar]
- Snary D., Goodfellow P., Bodmer W. F., Crumpton M. J. Evidence against a dimeric structure for membrane-bound HLA antigens. Nature. 1975 Nov 20;258(5532):240–242. doi: 10.1038/258240a0. [DOI] [PubMed] [Google Scholar]
- Springer T. A., Mann D. L., DeFranco A. L., Strominger J. L. Detergent solubilization, purification, and separation of specificities of HLA antigens from a cultured human lymphoblastoid line, RPMI 4265. J Biol Chem. 1977 Jul 10;252(13):4682–4693. [PubMed] [Google Scholar]
- Trimble R. B., Tarentino A. L., Plummer T. H., Jr, Maley F. Asparaginyl glycopeptides with a low mannose content are hydrolyzed by endo-beta-N-acetylglucosaminidase H. J Biol Chem. 1978 Jul 10;253(13):4508–4511. [PubMed] [Google Scholar]
- Waechter C. J., Lennarz W. J. The role of polyprenol-linked sugars in glycoprotein synthesis. Annu Rev Biochem. 1976;45:95–112. doi: 10.1146/annurev.bi.45.070176.000523. [DOI] [PubMed] [Google Scholar]