Abstract
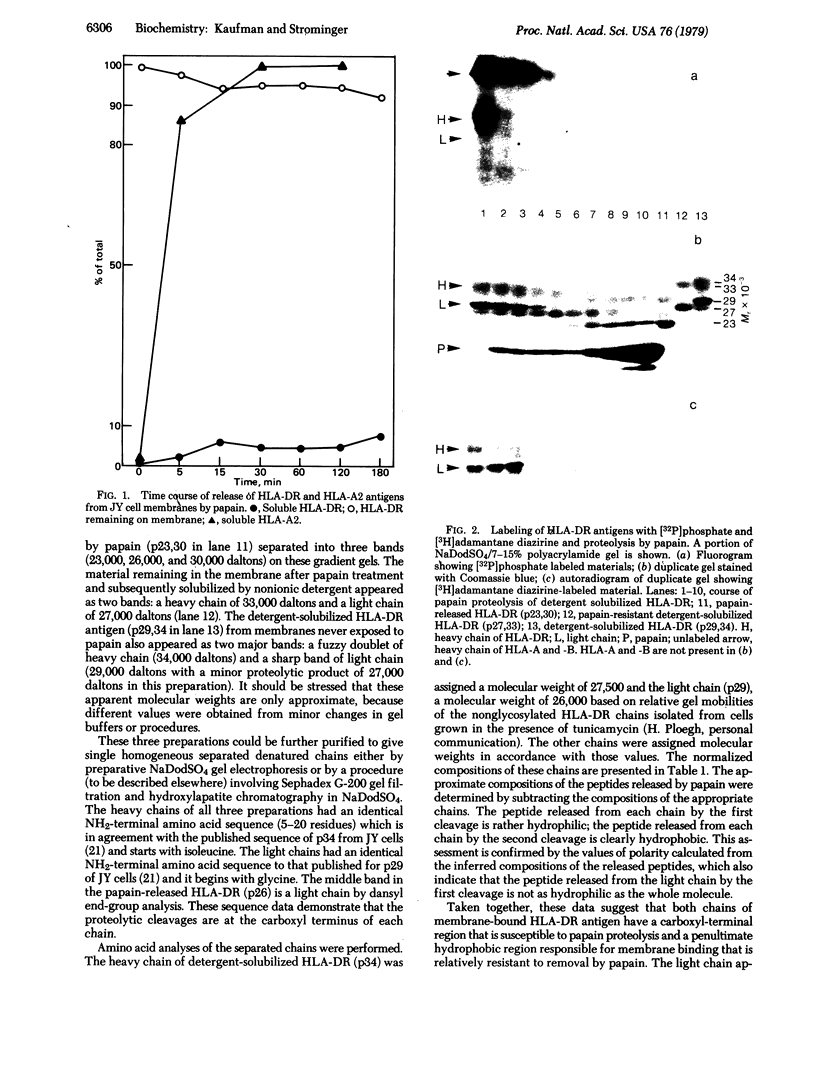
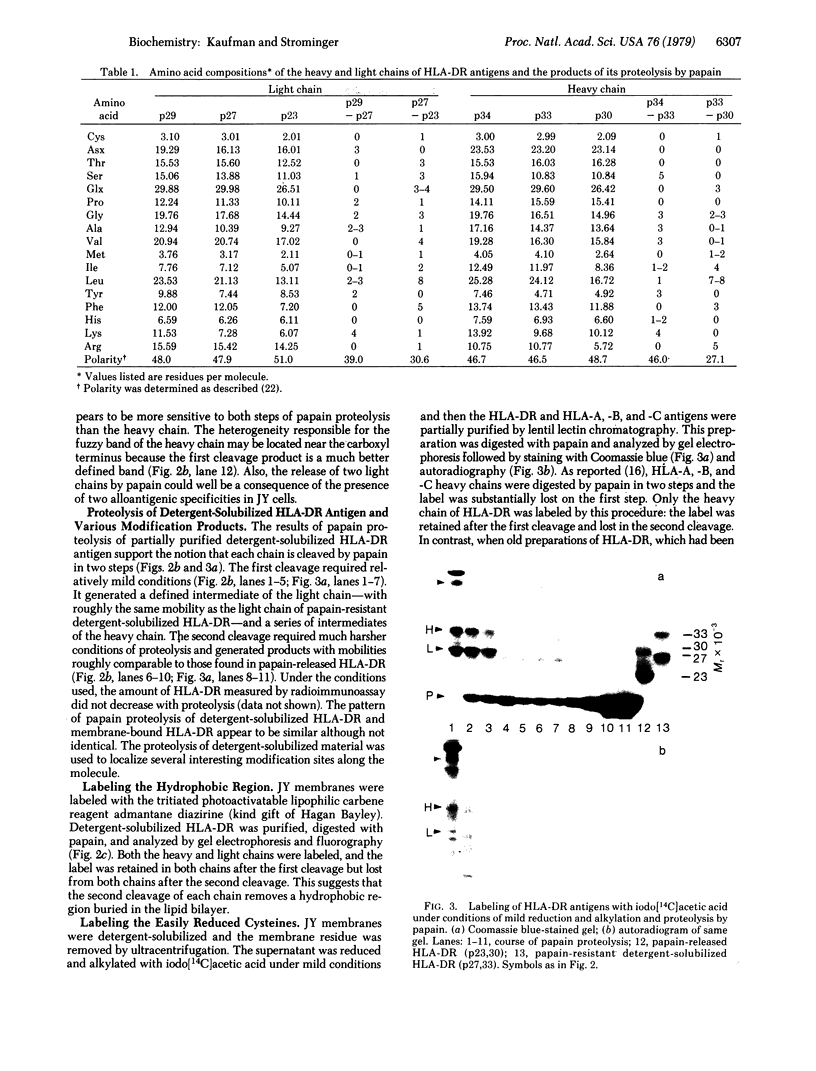
The HLA-DR antigen, a complex of two glycoproteins of 29,000 and 34,000 daltons, can be isolated from the membranes of human B-lymphoblastoid cell lines. Extensive proteolysis releases only 5-10% of the antigen, whereas detergent solubilizes all of it. Detergent solubilization after papain proteolysis of membranes produces antigen with chains cleaved near the carboxyl termini. Comparison of these three preparations demonstrates that each chain contains a carboxyl-terminal hydrophilic region that is sensitive to proteolytic degradation and a penultimate hydrophobic region, responsible for membrane binding, that is more resistant to papain. This two-step cleavage of each chain is also observed during the proteolysis of detergent-solubilized HLA-DR antigen. Both chains of HLA-DR in the membrane can be labeled with the lipophilic photoactivatable carbene reagent adamantane diazirine. This label is released from both chains during the second cleavage. The heavy chain can be reduced and alkylated under mild conditions, and this label is also lost during the second cleavage. The heavy chain is phosphorylated in vivo, and this label is lost upon the first cleavage. This observation suggests that the carboxyl terminus of the heavy chain is intracellular. Cumulatively, these data suggest that both chains of HLA-DR antigens are comprised of large extracellular NH2-terminal regions, small penultimate intramembranous regions, and small carboxyl-terminal intracellular regions.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bonner W. M., Laskey R. A. A film detection method for tritium-labelled proteins and nucleic acids in polyacrylamide gels. Eur J Biochem. 1974 Jul 1;46(1):83–88. doi: 10.1111/j.1432-1033.1974.tb03599.x. [DOI] [PubMed] [Google Scholar]
- Capaldi R. A., Vanderkooi G. The low polarity of many membrane proteins. Proc Natl Acad Sci U S A. 1972 Apr;69(4):930–932. doi: 10.1073/pnas.69.4.930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrone S., Allison J. P., Pellegrino M. A. Human DR (Ia-like) antigens: biological and molecular profile. Contemp Top Mol Immunol. 1978;7:239–281. doi: 10.1007/978-1-4757-0779-3_8. [DOI] [PubMed] [Google Scholar]
- Goldman D. W., Pober J. S., White J., Bayley H. Selective labelling of the hydrophobic segments of intrinsic membrane proteins with a lipophilic photogenerated carbene. Nature. 1979 Aug 30;280(5725):841–843. doi: 10.1038/280841a0. [DOI] [PubMed] [Google Scholar]
- Humphreys R. E., McCune J. M., Chess L., Herrman H. C., Malenka D. J., Mann D. L., Parham P., Schlossman S. F., Strominger J. L. Isolation and immunologic characterization of a human. B-lymphocyte-specific, cell surface antigen. J Exp Med. 1976 Jul 1;144(1):98–112. doi: 10.1084/jem.144.1.98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler S. W. Cell membrane antigen isolation with the staphylococcal protein A-antibody adsorbent. J Immunol. 1976 Nov;117(5 Pt 1):1482–1490. [PubMed] [Google Scholar]
- Klein J. The major histocompatibility complex of the mouse. Science. 1979 Feb 9;203(4380):516–521. doi: 10.1126/science.104386. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Marchesi V. T., Furthmayr H., Tomita M. The red cell membrane. Annu Rev Biochem. 1976;45:667–698. doi: 10.1146/annurev.bi.45.070176.003315. [DOI] [PubMed] [Google Scholar]
- Orr H. T., Lopez de Castro J. A., Parham P., Ploegh H. L., Strominger J. L. Comparison of amino acid sequences of two human histocompatibility antigens, HLA-A2 and HLA-B7: location of putative alloantigenic sites. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4395–4399. doi: 10.1073/pnas.76.9.4395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parham P., Alpert B. N., Orr H. T., Strominger J. L. Carbohydrate moiety of HLA antigens. Antigenic properties and amino acid sequences around the site of glycosylation. J Biol Chem. 1977 Nov 10;252(21):7555–7567. [PubMed] [Google Scholar]
- Pober J. S., Guild B. C., Strominger J. L. Phosphorylation in vivo and in vitro of human histocompatibility antigens (HLA-A and HLA-B) in the carboxy-terminal intracellular domain. Proc Natl Acad Sci U S A. 1978 Dec;75(12):6002–6006. doi: 10.1073/pnas.75.12.6002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robb R. J., Terhorst C., Strominger J. L. Sequence of the COOH-terminal hydrophilic region of histocompatibility antigens HLA-A2 and HLA-B7. J Biol Chem. 1978 Aug 10;253(15):5319–5324. [PubMed] [Google Scholar]
- Silver J., Ferrone S. Structural polymorphism of human DR antigens. Nature. 1979 May 31;279(5712):436–437. doi: 10.1038/279436a0. [DOI] [PubMed] [Google Scholar]
- Springer T. A., Kaufman J. F., Siddoway L. A., Mann D. L., Strominger J. L. Purification of HLA-linked B lymphocyte alloantigens in immunologically active form by preparative sodium dodecyl sulfate-gel electrophoresis and studies on their subunit association. J Biol Chem. 1977 Sep 10;252(17):6201–6207. [PubMed] [Google Scholar]
- Springer T. A., Kaufman J. F., Terhorst C., Strominger J. L. Purification and structural characterisation of human HLA-linked B-cell antigens. Nature. 1977 Jul 21;268(5617):213–218. doi: 10.1038/268213a0. [DOI] [PubMed] [Google Scholar]
- Springer T. A., Mann D. L., DeFranco A. L., Strominger J. L. Detergent solubilization, purification, and separation of specificities of HLA antigens from a cultured human lymphoblastoid line, RPMI 4265. J Biol Chem. 1977 Jul 10;252(13):4682–4693. [PubMed] [Google Scholar]
- Springer T. A., Strominger J. L. Detergent-soluble HLA antigens contain a hydrophilic region at the COOH-terminus and a penultimate hydrophobic region. Proc Natl Acad Sci U S A. 1976 Jul;73(7):2481–2485. doi: 10.1073/pnas.73.7.2481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh F. S., Crumpton M. J. Orientation of cell-surface antigens in the lipid bilayer of lymphocyte plasma membrane. Nature. 1977 Sep 22;269(5626):307–311. doi: 10.1038/269307a0. [DOI] [PubMed] [Google Scholar]
- Woods K. R., Wang K. T. Separation of dansyl-amino acids by polyamide layer chromatography. Biochim Biophys Acta. 1967 Feb 21;133(2):369–370. doi: 10.1016/0005-2795(67)90078-5. [DOI] [PubMed] [Google Scholar]