Abstract
Analysis of the kinetics of hybridization in liquid of labeled herpes simplex virus-1 DNA and excess viral RNA revealed the following: (i) Cells infected by herpes simplex virus-1 for 2 hr (before DNA synthesis) contain two classes of RNA molecules differing 140-fold in molar concentrations. The abundant and scarce RNAs are transcribed from 14 and 30% of the DNA, respectively. RNA extracted at 8 hr after infection (late RNA) also contains abundant and scarce classes differing 40-fold in molar concentrations; these are transcribed from 19 and 28% of viral DNA, respectively. Abundance competition hybridization tests indicate that the abundant RNA at 2 hr is a subset of the 8-hr abundant RNA. (ii) The abundant RNAs probably specify structural proteins, as indicated by estimates of DNA template required for structural proteins and by experiments showing that 19 of 24 proteins (corresponding to 68% of genetic information for structural proteins) are already made between 0.5 and 2 hr after infection. We conclude that there are two types of transcriptional controls, i.e., on-off and abundance controls, and that the synthesis of most structural components is an early viral function.
Keywords: hybridization, nucleases, mammalian cells
Full text
PDF
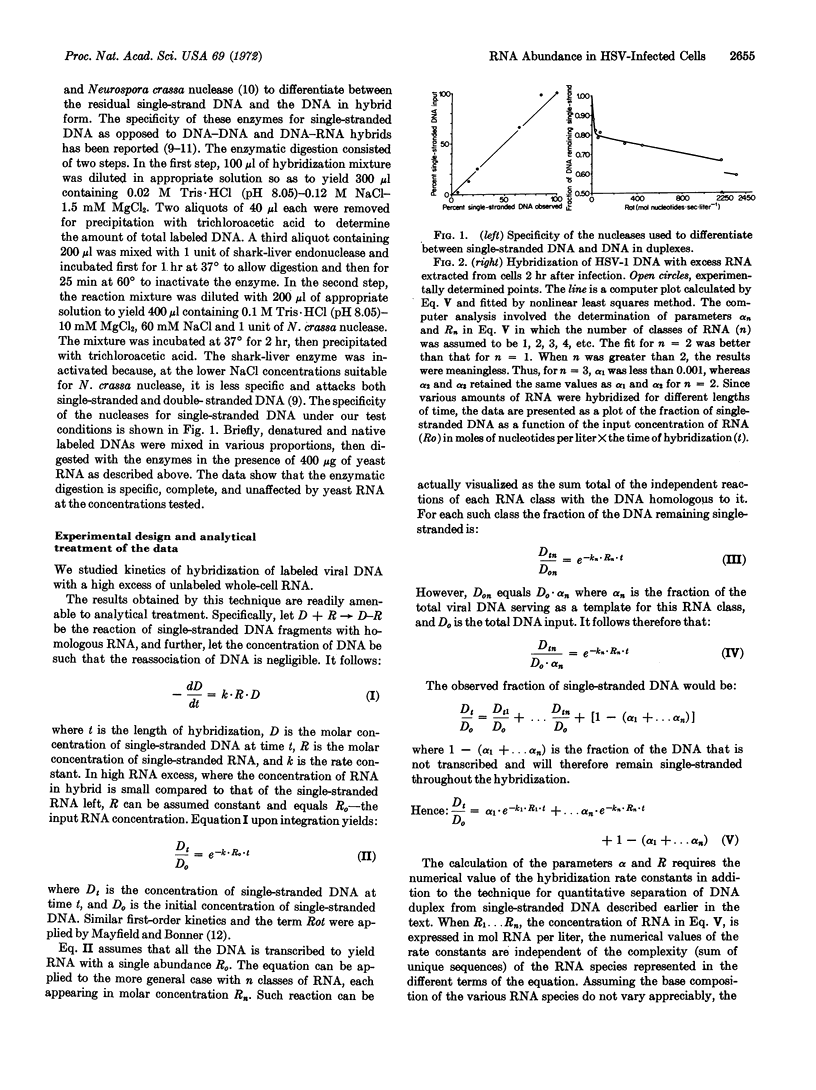
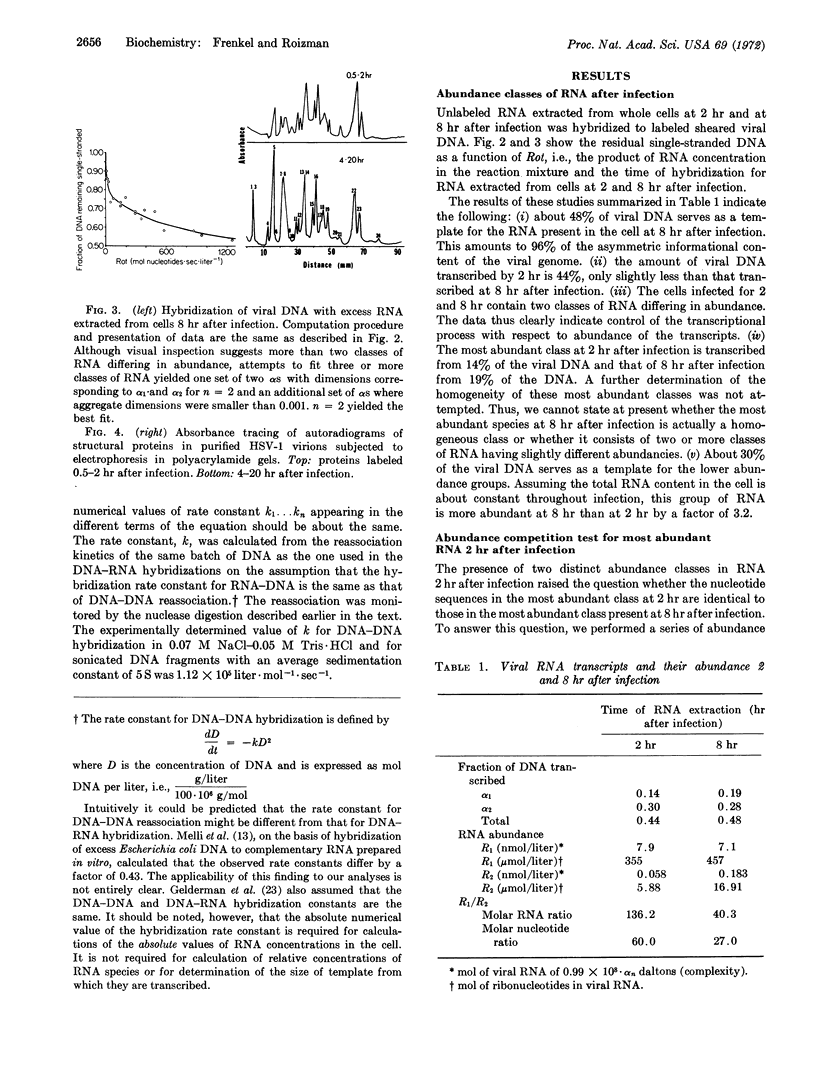


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ASHE H., SEAMAN E., VANVUNAKIS H., LEVINE L. CHARACTERIZATION OF A DEOXYRIBONUCLEASE OF MUSTELUS CANIS LIVER. Biochim Biophys Acta. 1965 May 18;99:298–306. doi: 10.1016/s0926-6593(65)80126-6. [DOI] [PubMed] [Google Scholar]
- Frenkel N., Roizman B. Herpes vimplex virus: genome size and redundancy studied by renaturation kinetics. J Virol. 1971 Oct;8(4):591–593. doi: 10.1128/jvi.8.4.591-593.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gelderman A. H., Rake A. V., Britten R. J. Transcription of nonrepeated DNA in neonatal and fetal mice. Proc Natl Acad Sci U S A. 1971 Jan;68(1):172–176. doi: 10.1073/pnas.68.1.172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gergely L., Klein G., Ernberg I. The action of DNA antagonists on Epstein-Barr virus (EBV)-associated early antigen (EA) in Burkitt lymphoma lines. Int J Cancer. 1971 Mar 15;7(2):293–302. doi: 10.1002/ijc.2910070214. [DOI] [PubMed] [Google Scholar]
- Kieff E. D., Bachenheimer S. L., Roizman B. Size, composition, and structure of the deoxyribonucleic acid of herpes simplex virus subtypes 1 and 2. J Virol. 1971 Aug;8(2):125–132. doi: 10.1128/jvi.8.2.125-132.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kieff E., Hoyer B., Bachenheimer S., Roizman B. Genetic relatedness of type 1 and type 2 herpes simplex viruses. J Virol. 1972 May;9(5):738–745. doi: 10.1128/jvi.9.5.738-745.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S. Y., Mendecki J., Brawerman G. A polynucleotide segment rich in adenylic acid in the rapidly-labeled polyribosomal RNA component of mouse sarcoma 180 ascites cells. Proc Natl Acad Sci U S A. 1971 Jun;68(6):1331–1335. doi: 10.1073/pnas.68.6.1331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manly K. F., Smoler D. F., Bromfeld E., Baltimore D. Forms of deoxyribonucleic acid produced by virions of the ribonucleic acid tumor viruses. J Virol. 1971 Jan;7(1):106–111. doi: 10.1128/jvi.7.1.106-111.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayfield J. E., Bonner J. Tissue differences in rat chromosomal RNA. Proc Natl Acad Sci U S A. 1971 Nov;68(11):2652–2655. doi: 10.1073/pnas.68.11.2652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melli M., Whitfield C., Rao K. V., Richardson M., Bishop J. O. DNA-RNA hybridization in vast DNA excess. Nat New Biol. 1971 May 5;231(18):8–12. [PubMed] [Google Scholar]
- O'Callaghan D. J., Hyde J. M., Gentry G. A., Randall C. C. Kinetics of viral deoxyribonucleic acid, protein, and infectious particle production and alterations in host macromolecular syntheses in equine abortion (herpes) virus-infected cells. J Virol. 1968 Aug;2(8):793–804. doi: 10.1128/jvi.2.8.793-804.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabin E. Z., Preiss B., Fraser M. J. A nuclease from Neurospora crassa conidia specific for single-stranded nucleic acids. Prep Biochem. 1971;1(4):283–307. doi: 10.1080/00327487108081946. [DOI] [PubMed] [Google Scholar]
- Roizman B., Spear P. G. Preparation of herpes simplex virus of high titer. J Virol. 1968 Jan;2(1):83–84. doi: 10.1128/jvi.2.1.83-84.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spear P. G., Roizman B. Proteins specified by herpes simplex virus, IV. Site of glycosylation and accumulation of viral membrane proteins. Proc Natl Acad Sci U S A. 1970 Jul;66(3):730–737. doi: 10.1073/pnas.66.3.730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spear P. G., Roizman B. Proteins specified by herpes simplex virus. V. Purification and structural proteins of the herpesvirion. J Virol. 1972 Jan;9(1):143–159. doi: 10.1128/jvi.9.1.143-159.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]


