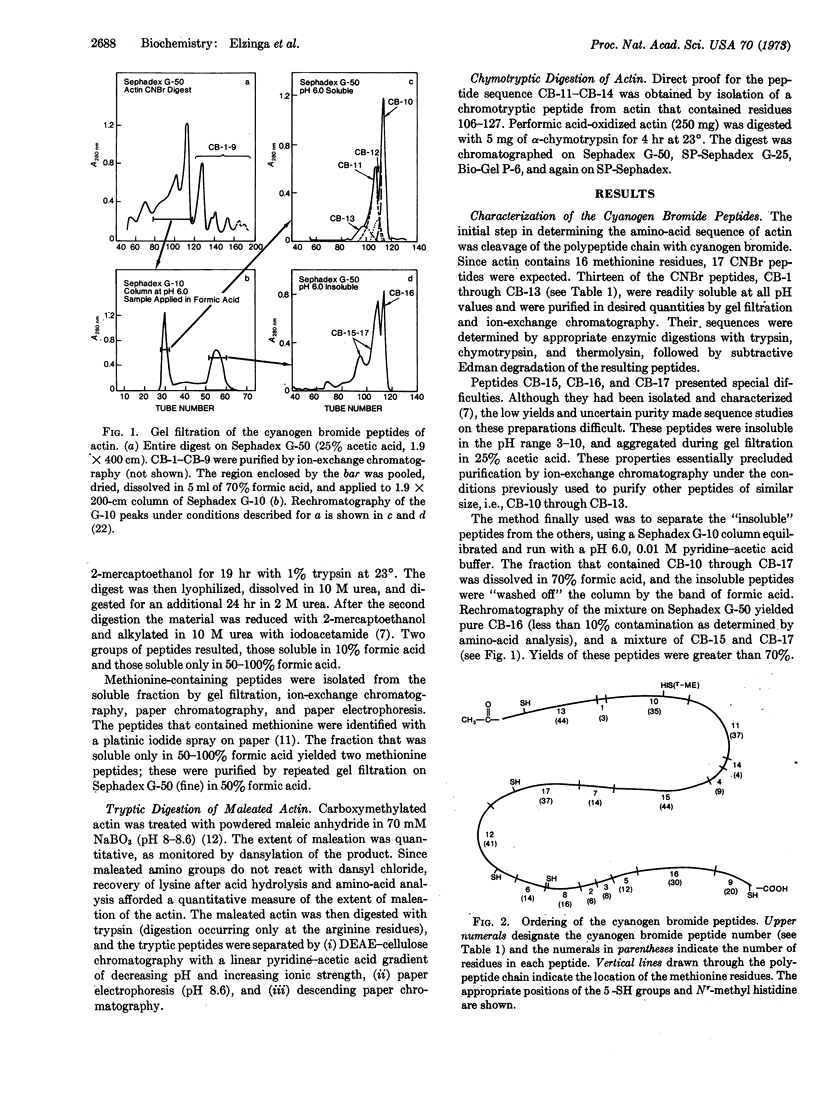
Abstract
The complete amino-acid sequence of actin of rabbit skeletal muscle was determined. The actin polypeptide chain is composed of 374 residues, including one residue of the unusual amino acid Nr-methyl histidine, and has a calculated molecular weight of 41,785. The sequence of actin was determined by isolating the peptides produced by cleavage of the protein with cyanogen bromide, determining the sequence of these peptides, and establishing their order within the molecule. This study represents the first complete determination of the aminoacid sequence of a myofibrillar protein. Comparison of this sequence with peptides from actins isolated from different sources indicates that the sequence of actin is highly conserved.
Keywords: cyanogen bromide cleavage, maleation, myofibrillar protein, Nr-methyl histidine
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adelstein R. S., Kuehl W. M. Structural studies on rabbit skeletal actin. I. Isolation and characterization of the peptides produced by cyanogen bromide cleavage. Biochemistry. 1970 Mar 17;9(6):1355–1364. doi: 10.1021/bi00808a009. [DOI] [PubMed] [Google Scholar]
- Asatoor A. M., Armstrong M. D. 3-methylhistidine, a component of actin. Biochem Biophys Res Commun. 1967 Jan 23;26(2):168–174. doi: 10.1016/0006-291x(67)90229-x. [DOI] [PubMed] [Google Scholar]
- Berl S., Puszkin S., Nicklas W. J. Actomyosin-like protein in brain. Science. 1973 Feb 2;179(4072):441–446. doi: 10.1126/science.179.4072.441. [DOI] [PubMed] [Google Scholar]
- Bridgen J. The amino acid sequence around four cysteine residues in trout actin. Biochem J. 1971 Jul;123(4):591–600. doi: 10.1042/bj1230591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bridgen J. The reactivity and function of thiol groups in trout actin. Biochem J. 1972 Jan;126(1):21–25. doi: 10.1042/bj1260021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler P. J., Harris J. I., Hartley B. S., Lebeman R. The use of maleic anhydride for the reversible blocking of amino groups in polypeptide chains. Biochem J. 1969 May;112(5):679–689. doi: 10.1042/bj1120679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elzinga M. Amino acid sequence aroung 3-methylhistidine in rabbit skeletal muscle actin. Biochemistry. 1971 Jan 19;10(2):224–229. doi: 10.1021/bi00778a005. [DOI] [PubMed] [Google Scholar]
- Elzinga M. Amino acid sequence studies on rabbit skeletal muscle actin. Cyanogen bromide cleavage of the protein and determination of the sequences of seven of the resulting peptides. Biochemistry. 1970 Mar 17;9(6):1365–1374. doi: 10.1021/bi00808a010. [DOI] [PubMed] [Google Scholar]
- Hardy M. F., Perry S. V. In vitro methylation of muscle proteins. Nature. 1969 Jul 19;223(5203):300–302. doi: 10.1038/223300a0. [DOI] [PubMed] [Google Scholar]
- Johnson P., Perry S. V. Chemical studies on the cysteine and terminal peptides in tryptic digests of actin. Biochem J. 1968 Nov;110(2):207–216. doi: 10.1042/bj1100207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laursen R. A., Horn M. J., Bonner A. G. Solid-phase Edman degradation. The use of p-phenyl diisothiocyanate to attach lysine- and arginine-containing peptides to insoluble resins. FEBS Lett. 1972 Mar;21(1):67–70. doi: 10.1016/0014-5793(72)80165-0. [DOI] [PubMed] [Google Scholar]
- Laursen R. A. Solid-phase Edman degradation. An automatic peptide sequencer. Eur J Biochem. 1971 May 11;20(1):89–102. doi: 10.1111/j.1432-1033.1971.tb01366.x. [DOI] [PubMed] [Google Scholar]
- Lusty C. J., Fasold H. Characterization of sulfhydryl groups of actin. Biochemistry. 1969 Jul;8(7):2933–2939. doi: 10.1021/bi00835a036. [DOI] [PubMed] [Google Scholar]
- Rees M. K., Young M. Studies on the isolation and molecular properties of homogeneous globular actin. Evidence for a single polypeptide chain structure. J Biol Chem. 1967 Oct 10;242(19):4449–4458. [PubMed] [Google Scholar]
- Weihing R. R., Korn E. D. Acanthamoeba actin. Composition of the peptide that contains 3-methylhistidine and a peptide that contains N e -methyllysine. Biochemistry. 1972 Apr 11;11(8):1538–1543. doi: 10.1021/bi00758a032. [DOI] [PubMed] [Google Scholar]
- Yang Y. Z., Perdue J. F. Contractile proteins of cultured cells. I. The isolation and characterization of an actin-like protein from cultured chick embryo fibroblasts. J Biol Chem. 1972 Jul 25;247(14):4503–4509. [PubMed] [Google Scholar]


