Abstract
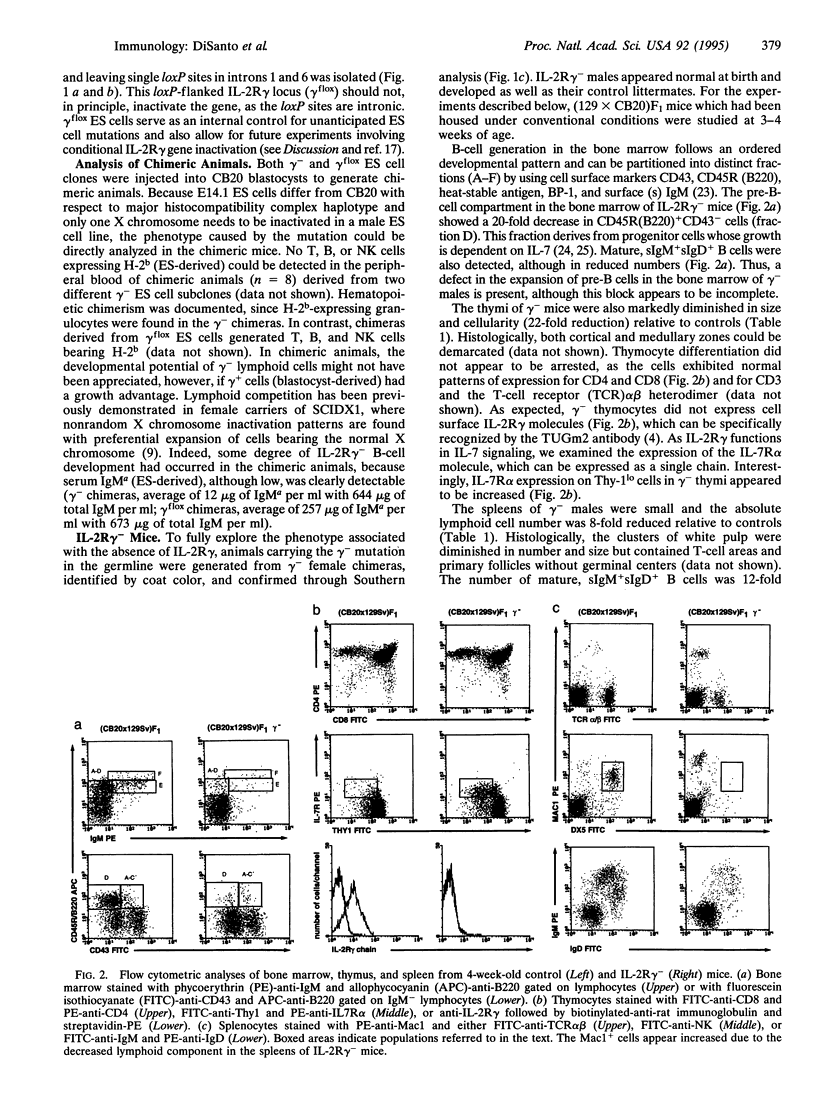
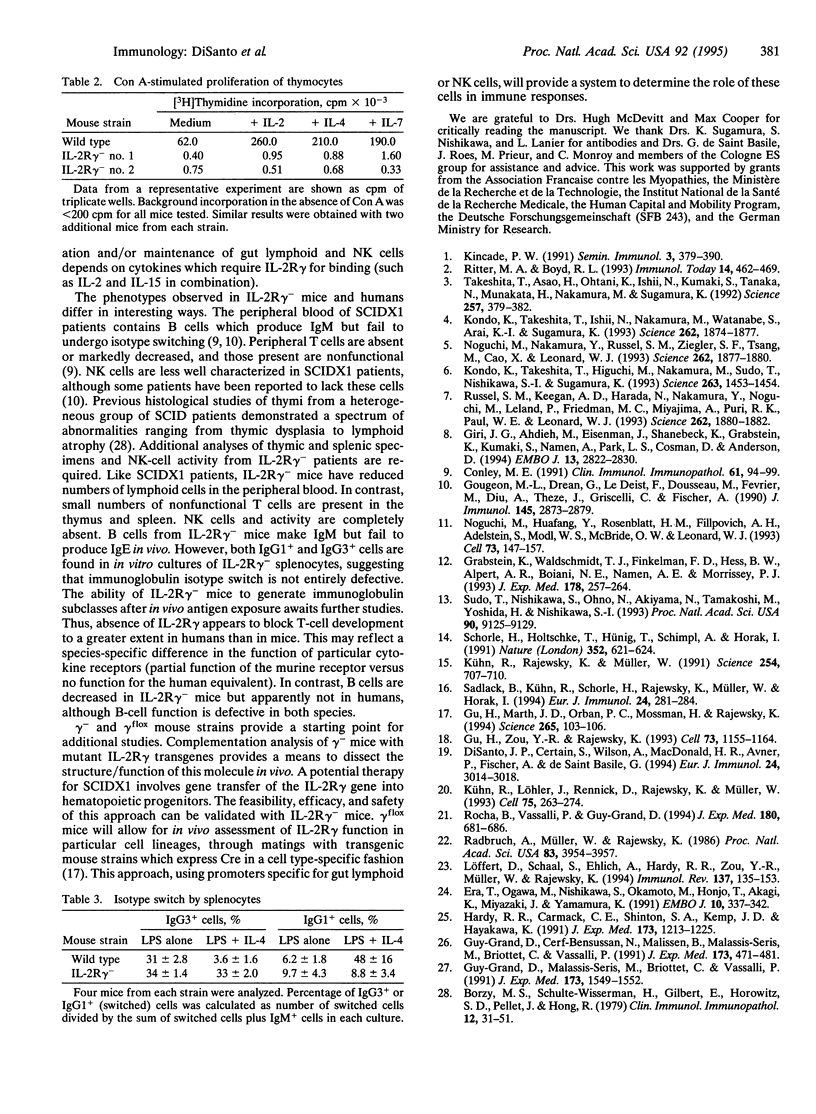
The interleukin 2 receptor gamma chain (IL-2R gamma) is a component of the receptors for IL-2, IL-4, IL-7, and IL-15. Mutations in IL-2R gamma in man appear responsible for the X chromosome-linked immunodeficiency SCIDX1, characterized by a defect in T-cell and natural killer (NK)-cell differentiation with the presence of poorly functioning B cells. To explore at which level IL-2R gamma affects lymphoid development in vivo, we have analyzed mice derived from embryonic stem (ES) cells with mutant IL-2R gamma loci generated by Cre/loxP-mediated recombination. In the peripheral blood of chimeric animals, lymphoid cells derived from IL-2R gamma- ES cells were not detected, although control ES cells carrying an IL-2R gamma gene with embedded loxP sites gave rise to T-, B-, and NK-cell lineages. Germline IL-2R gamma-deficient male animals, however, developed some mature splenic B and T cells, although the absolute number of lymphocytes was almost 10-fold reduced. In contrast, there was a complete disappearance of NK cells (over 350-fold reduction). Development of gut-associated intraepithelial lymphocytes was also severely diminished, and Peyer's patches were not detected. In vitro mitogenic responses of thymocytes, IL-4-directed immunoglobulin class switch of splenocytes, and NK activity were defective. Thus, IL-2R gamma facilitates mainstream B- and T-cell generation and function and also appears to be essential for NK-cell development.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Borzy M. S., Schulte-Wissermann H., Gilbert E., Horowitz S. D., Pellett J., Hong R. Thymic morphology in immunodeficiency diseases: results of thymic biopsies. Clin Immunol Immunopathol. 1979 Jan;12(1):31–51. doi: 10.1016/0090-1229(79)90109-0. [DOI] [PubMed] [Google Scholar]
- DiSanto J. P., Certain S., Wilson A., MacDonald H. R., Avner P., Fischer A., de Saint Basile G. The murine interleukin-2 receptor gamma chain gene: organization, chromosomal localization and expression in the adult thymus. Eur J Immunol. 1994 Dec;24(12):3014–3018. doi: 10.1002/eji.1830241214. [DOI] [PubMed] [Google Scholar]
- Era T., Ogawa M., Nishikawa S., Okamoto M., Honjo T., Akagi K., Miyazaki J., Yamamura K. Differentiation of growth signal requirement of B lymphocyte precursor is directed by expression of immunoglobulin. EMBO J. 1991 Feb;10(2):337–342. doi: 10.1002/j.1460-2075.1991.tb07954.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giri J. G., Ahdieh M., Eisenman J., Shanebeck K., Grabstein K., Kumaki S., Namen A., Park L. S., Cosman D., Anderson D. Utilization of the beta and gamma chains of the IL-2 receptor by the novel cytokine IL-15. EMBO J. 1994 Jun 15;13(12):2822–2830. doi: 10.1002/j.1460-2075.1994.tb06576.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gougeon M. L., Drean G., Le Deist F., Dousseau M., Fevrier M., Diu A., Theze J., Griscelli C., Fischer A. Human severe combined immunodeficiency disease: phenotypic and functional characteristics of peripheral B lymphocytes. J Immunol. 1990 Nov 1;145(9):2873–2879. [PubMed] [Google Scholar]
- Grabstein K. H., Waldschmidt T. J., Finkelman F. D., Hess B. W., Alpert A. R., Boiani N. E., Namen A. E., Morrissey P. J. Inhibition of murine B and T lymphopoiesis in vivo by an anti-interleukin 7 monoclonal antibody. J Exp Med. 1993 Jul 1;178(1):257–264. doi: 10.1084/jem.178.1.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu H., Marth J. D., Orban P. C., Mossmann H., Rajewsky K. Deletion of a DNA polymerase beta gene segment in T cells using cell type-specific gene targeting. Science. 1994 Jul 1;265(5168):103–106. doi: 10.1126/science.8016642. [DOI] [PubMed] [Google Scholar]
- Gu H., Zou Y. R., Rajewsky K. Independent control of immunoglobulin switch recombination at individual switch regions evidenced through Cre-loxP-mediated gene targeting. Cell. 1993 Jun 18;73(6):1155–1164. doi: 10.1016/0092-8674(93)90644-6. [DOI] [PubMed] [Google Scholar]
- Guy-Grand D., Cerf-Bensussan N., Malissen B., Malassis-Seris M., Briottet C., Vassalli P. Two gut intraepithelial CD8+ lymphocyte populations with different T cell receptors: a role for the gut epithelium in T cell differentiation. J Exp Med. 1991 Feb 1;173(2):471–481. doi: 10.1084/jem.173.2.471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guy-Grand D., Malassis-Seris M., Briottet C., Vassalli P. Cytotoxic differentiation of mouse gut thymodependent and independent intraepithelial T lymphocytes is induced locally. Correlation between functional assays, presence of perforin and granzyme transcripts, and cytoplasmic granules. J Exp Med. 1991 Jun 1;173(6):1549–1552. doi: 10.1084/jem.173.6.1549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardy R. R., Carmack C. E., Shinton S. A., Kemp J. D., Hayakawa K. Resolution and characterization of pro-B and pre-pro-B cell stages in normal mouse bone marrow. J Exp Med. 1991 May 1;173(5):1213–1225. doi: 10.1084/jem.173.5.1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kincade P. W. Molecular interactions between stromal cells and B lymphocyte precursors. Semin Immunol. 1991 Nov;3(6):379–390. [PubMed] [Google Scholar]
- Kondo M., Takeshita T., Higuchi M., Nakamura M., Sudo T., Nishikawa S., Sugamura K. Functional participation of the IL-2 receptor gamma chain in IL-7 receptor complexes. Science. 1994 Mar 11;263(5152):1453–1454. doi: 10.1126/science.8128231. [DOI] [PubMed] [Google Scholar]
- Kondo M., Takeshita T., Ishii N., Nakamura M., Watanabe S., Arai K., Sugamura K. Sharing of the interleukin-2 (IL-2) receptor gamma chain between receptors for IL-2 and IL-4. Science. 1993 Dec 17;262(5141):1874–1877. doi: 10.1126/science.8266076. [DOI] [PubMed] [Google Scholar]
- Kühn R., Löhler J., Rennick D., Rajewsky K., Müller W. Interleukin-10-deficient mice develop chronic enterocolitis. Cell. 1993 Oct 22;75(2):263–274. doi: 10.1016/0092-8674(93)80068-p. [DOI] [PubMed] [Google Scholar]
- Kühn R., Rajewsky K., Müller W. Generation and analysis of interleukin-4 deficient mice. Science. 1991 Nov 1;254(5032):707–710. doi: 10.1126/science.1948049. [DOI] [PubMed] [Google Scholar]
- Löffert D., Schaal S., Ehlich A., Hardy R. R., Zou Y. R., Müller W., Rajewsky K. Early B-cell development in the mouse: insights from mutations introduced by gene targeting. Immunol Rev. 1994 Feb;137:135–153. doi: 10.1111/j.1600-065x.1994.tb00662.x. [DOI] [PubMed] [Google Scholar]
- Noguchi M., Nakamura Y., Russell S. M., Ziegler S. F., Tsang M., Cao X., Leonard W. J. Interleukin-2 receptor gamma chain: a functional component of the interleukin-7 receptor. Science. 1993 Dec 17;262(5141):1877–1880. doi: 10.1126/science.8266077. [DOI] [PubMed] [Google Scholar]
- Noguchi M., Yi H., Rosenblatt H. M., Filipovich A. H., Adelstein S., Modi W. S., McBride O. W., Leonard W. J. Interleukin-2 receptor gamma chain mutation results in X-linked severe combined immunodeficiency in humans. Cell. 1993 Apr 9;73(1):147–157. doi: 10.1016/0092-8674(93)90167-o. [DOI] [PubMed] [Google Scholar]
- Radbruch A., Müller W., Rajewsky K. Class switch recombination is IgG1 specific on active and inactive IgH loci of IgG1-secreting B-cell blasts. Proc Natl Acad Sci U S A. 1986 Jun;83(11):3954–3957. doi: 10.1073/pnas.83.11.3954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritter M. A., Boyd R. L. Development in the thymus: it takes two to tango. Immunol Today. 1993 Sep;14(9):462–469. doi: 10.1016/0167-5699(93)90250-O. [DOI] [PubMed] [Google Scholar]
- Rocha B., Vassalli P., Guy-Grand D. Thymic and extrathymic origins of gut intraepithelial lymphocyte populations in mice. J Exp Med. 1994 Aug 1;180(2):681–686. doi: 10.1084/jem.180.2.681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell S. M., Keegan A. D., Harada N., Nakamura Y., Noguchi M., Leland P., Friedmann M. C., Miyajima A., Puri R. K., Paul W. E. Interleukin-2 receptor gamma chain: a functional component of the interleukin-4 receptor. Science. 1993 Dec 17;262(5141):1880–1883. doi: 10.1126/science.8266078. [DOI] [PubMed] [Google Scholar]
- Sadlack B., Kühn R., Schorle H., Rajewsky K., Müller W., Horak I. Development and proliferation of lymphocytes in mice deficient for both interleukins-2 and -4. Eur J Immunol. 1994 Jan;24(1):281–284. doi: 10.1002/eji.1830240144. [DOI] [PubMed] [Google Scholar]
- Schorle H., Holtschke T., Hünig T., Schimpl A., Horak I. Development and function of T cells in mice rendered interleukin-2 deficient by gene targeting. Nature. 1991 Aug 15;352(6336):621–624. doi: 10.1038/352621a0. [DOI] [PubMed] [Google Scholar]
- Sudo T., Nishikawa S., Ohno N., Akiyama N., Tamakoshi M., Yoshida H., Nishikawa S. Expression and function of the interleukin 7 receptor in murine lymphocytes. Proc Natl Acad Sci U S A. 1993 Oct 1;90(19):9125–9129. doi: 10.1073/pnas.90.19.9125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeshita T., Asao H., Ohtani K., Ishii N., Kumaki S., Tanaka N., Munakata H., Nakamura M., Sugamura K. Cloning of the gamma chain of the human IL-2 receptor. Science. 1992 Jul 17;257(5068):379–382. doi: 10.1126/science.1631559. [DOI] [PubMed] [Google Scholar]




