Abstract
Several properties of the viral RNA-dependent DNA polymerases and of rabbit globin mRNA make it possible to consider synthesis of the globin gene in vitro. These enzymes copy an RNA template using a short sequence of complementary nucleotides as a primer. Furthermore, globin mRNA has a 3′-terminal sequence of adenylic acid residues that make it particularly suitable as a template, since oligo(dT) can be annealed to a specific site on the mRNA. This small primer could phase the DNA polymerase, possibly ensuring that replication is initiated from that end of the globin message. We have used this approach and find that purified mRNA is an efficient template for the polymerase enzyme. The reaction requires the RNA template and the four deoxyribonucleoside triphosphates, and it is markedly stimulated by the addition of oligo(dT). Consistent with the expectation that the oligo(dT) uniquely phases the polymerase at an adenine-rich region in the globin message, oligo(dG), oligo(dC), and oligo(dA) fail to serve as primers. The product has a density intermediate between that of DNA and RNA, and shifts to a lighter DNA density after treatment with base. Further, it is specifically complementary to globin mRNA and sediments slightly faster in an alkaline sucrose gradient than a DNA standard that has a molecular weight of 129,000. The data suggest that a major portion of the DNA product is a sequence of at least 500 bases, about 50 more than would be necessary to encode rabbit globin. The potential usefulness of this interesting product is discussed.
Keywords: RNA-dependent DNA polymerase, reticulocyte, hemoglobin, density gradient centrifugation, oligo(dT) primer
Full text
PDF
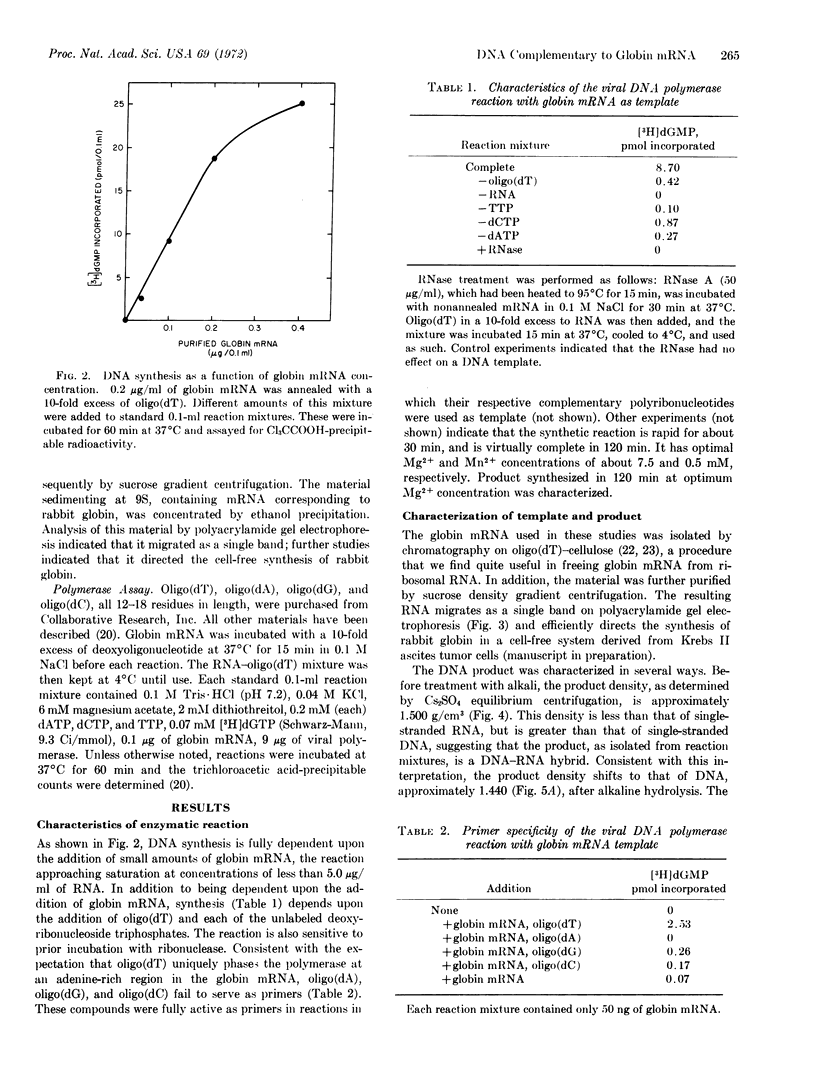
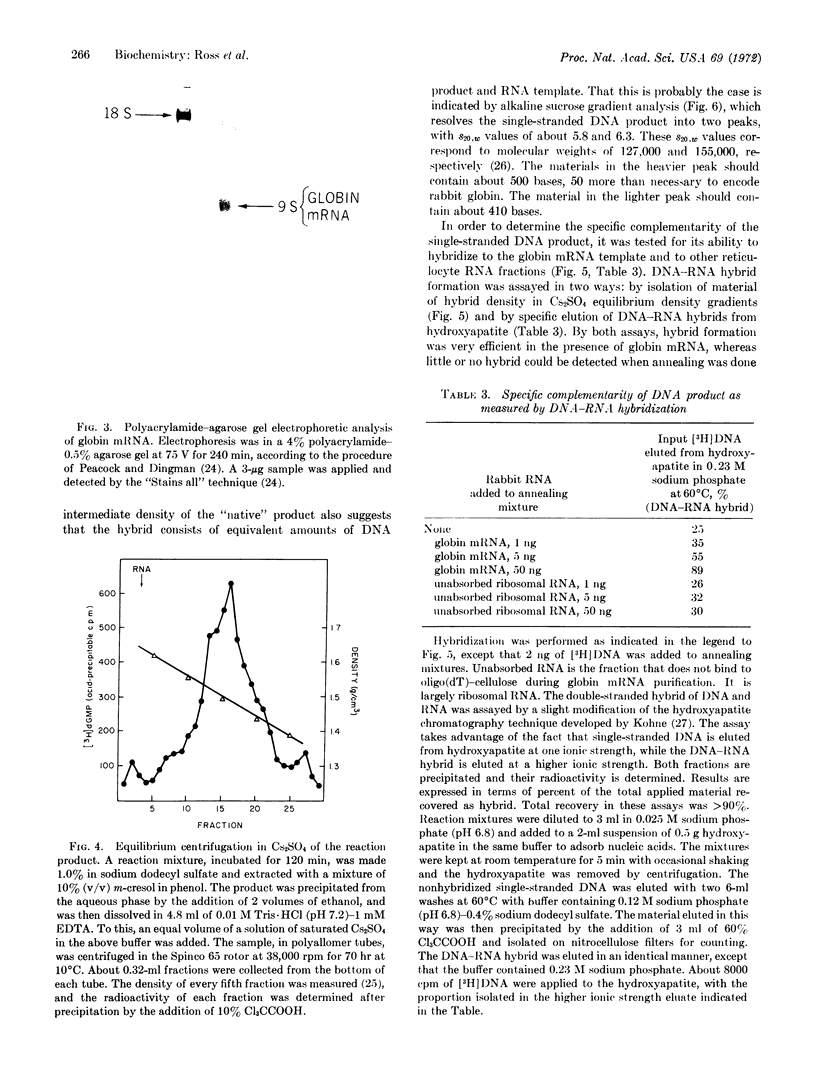
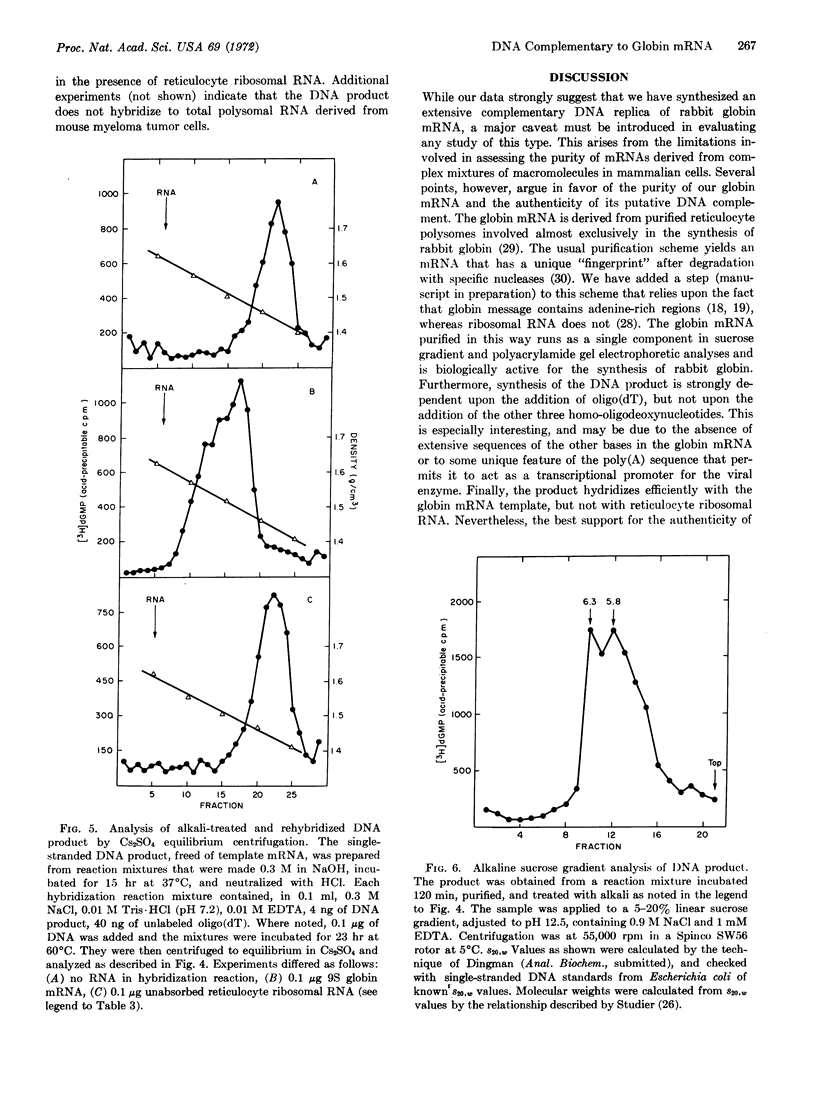

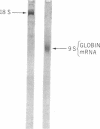
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baltimore D. RNA-dependent DNA polymerase in virions of RNA tumour viruses. Nature. 1970 Jun 27;226(5252):1209–1211. doi: 10.1038/2261209a0. [DOI] [PubMed] [Google Scholar]
- Baltimore D., Smoler D. Primer requirement and template specificity of the DNA polymerase of RNA tumor viruses. Proc Natl Acad Sci U S A. 1971 Jul;68(7):1507–1511. doi: 10.1073/pnas.68.7.1507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burr H., Lingrel J. B. Poly A sequences at the 3' termini of rabbit globin mRNAs. Nat New Biol. 1971 Sep 8;233(36):41–43. doi: 10.1038/newbio233041a0. [DOI] [PubMed] [Google Scholar]
- Darnell J. E., Wall R., Tushinski R. J. An adenylic acid-rich sequence in messenger RNA of HeLa cells and its possible relationship to reiterated sites in DNA. Proc Natl Acad Sci U S A. 1971 Jun;68(6):1321–1325. doi: 10.1073/pnas.68.6.1321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duesberg P., Helm K. V., Canaani E. Comparative properties of RNA and DNA templates for the DNA polymerase of Rous sarcoma virus. Proc Natl Acad Sci U S A. 1971 Oct;68(10):2505–2509. doi: 10.1073/pnas.68.10.2505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edmonds M., Caramela M. G. The isolation and characterization of adenosine monophosphate-rich polynucleotides synthesized by Ehrlich ascites cells. J Biol Chem. 1969 Mar 10;244(5):1314–1324. [PubMed] [Google Scholar]
- Edmonds M., Vaughan M. H., Jr, Nakazato H. Polyadenylic acid sequences in the heterogeneous nuclear RNA and rapidly-labeled polyribosomal RNA of HeLa cells: possible evidence for a precursor relationship. Proc Natl Acad Sci U S A. 1971 Jun;68(6):1336–1340. doi: 10.1073/pnas.68.6.1336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujinaga K., Parsons J. T., Beard J. W., Beard D., Green M. Mechanism of carcinogenesis by RNA tumor viruses. 3. Formation of RNA, DNA complex and duplex DNA molecules by the DNA polymerase (s) of avian myeloblastosis virus. Proc Natl Acad Sci U S A. 1970 Nov;67(3):1432–1439. doi: 10.1073/pnas.67.3.1432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garapin A. C., McDonnell J. P., Levinson W., Quintrell N., Fanshier L., Bishop J. M. Deoxyribonucleic acid polymerase associated with Rous sarcoma virus and avian myeloblastosis virus: properties of the enzyme and its product. J Virol. 1970 Nov;6(5):589–598. doi: 10.1128/jvi.6.5.589-598.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaskill P., Kabat D. Unexpectedly large size of globin messenger ribonucleic acid. Proc Natl Acad Sci U S A. 1971 Jan;68(1):72–75. doi: 10.1073/pnas.68.1.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green M., Rokutanda M., Fujinaga K., Ray R. K., Rokutanda H., Gurgo C. Mechanism of carcinogenesis by RNA tumor viruses. I. An RNA-dependent DNA polymerase in murine sarcoma viruses. Proc Natl Acad Sci U S A. 1970 Sep;67(1):385–393. doi: 10.1073/pnas.67.1.385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatanaka M., Huebner R. J., Gilden R. V. DNA polymerase activity associated with RNA tumor viruses. Proc Natl Acad Sci U S A. 1970 Sep;67(1):143–147. doi: 10.1073/pnas.67.1.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohne D. E. Isolation and characterization of bacterial ribosomal RNA cistrons. Biophys J. 1968 Oct;8(10):1104–1118. doi: 10.1016/S0006-3495(68)86542-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Labrie F. Isolation of an RNA with the properties of haemoglobin messenger. Nature. 1969 Mar 29;221(5187):1217–1222. doi: 10.1038/2211217a0. [DOI] [PubMed] [Google Scholar]
- Lee S. Y., Mendecki J., Brawerman G. A polynucleotide segment rich in adenylic acid in the rapidly-labeled polyribosomal RNA component of mouse sarcoma 180 ascites cells. Proc Natl Acad Sci U S A. 1971 Jun;68(6):1331–1335. doi: 10.1073/pnas.68.6.1331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim L., Canellakis E. S. Adenine-rich polymer associated with rabbit reticulocyte messenger RNA. Nature. 1970 Aug 15;227(5259):710–712. doi: 10.1038/227710a0. [DOI] [PubMed] [Google Scholar]
- McDonnell J. P., Garapin A. C., Levinson W. E., Quintrell N., Fanshier L., Bishop J. M. DNA polymerases of Rous sarcoma virus: delineation of two reactions with actinomycin. Nature. 1970 Oct 31;228(5270):433–435. doi: 10.1038/228433a0. [DOI] [PubMed] [Google Scholar]
- Mizutani S., Boettiger D., Temin H. M. A DNA-depenent DNA polymerase and a DNA endonuclease in virions of Rous sarcoma virus. Nature. 1970 Oct 31;228(5270):424–427. doi: 10.1038/228424a0. [DOI] [PubMed] [Google Scholar]
- Peacock A. C., Dingman C. W. Molecular weight estimation and separation of ribonucleic acid by electrophoresis in agarose-acrylamide composite gels. Biochemistry. 1968 Feb;7(2):668–674. doi: 10.1021/bi00842a023. [DOI] [PubMed] [Google Scholar]
- Ross J., Scolnick E. M., Todaro G. J., Aaronson S. A. Separation of murine cellular and murine leukaemia virus DNA polymerases. Nat New Biol. 1971 Jun 9;231(23):163–167. doi: 10.1038/newbio231163a0. [DOI] [PubMed] [Google Scholar]
- Ríman J., Beaudreau G. S. Viral DNA-dependent DNA polymerase and the properties of thymidine labelled material in virions of an oncogenic RNA virus. Nature. 1970 Oct 31;228(5270):427–430. doi: 10.1038/228427a0. [DOI] [PubMed] [Google Scholar]
- STUDIER F. W. SEDIMENTATION STUDIES OF THE SIZE AND SHAPE OF DNA. J Mol Biol. 1965 Feb;11:373–390. doi: 10.1016/s0022-2836(65)80064-x. [DOI] [PubMed] [Google Scholar]
- Schweet R., Lamfrom H., Allen E. THE SYNTHESIS OF HEMOGLOBIN IN A CELL-FREE SYSTEM. Proc Natl Acad Sci U S A. 1958 Oct 15;44(10):1029–1035. doi: 10.1073/pnas.44.10.1029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scolnick E. M., Aaronson S. A., Todaro G. J. DNA synthesis by RNA-containing tumor viruses. Proc Natl Acad Sci U S A. 1970 Oct;67(2):1034–1041. doi: 10.1073/pnas.67.2.1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spiegelman S., Burny A., Das M. R., Keydar J., Schlom J., Travnicek M., Watson K. Characterization of the products of DNA-directed DNA polymerases in oncogenic RNA viruses. Nature. 1970 Aug 8;227(5258):563–567. doi: 10.1038/227563a0. [DOI] [PubMed] [Google Scholar]
- Spiegelman S., Burny A., Das M. R., Keydar J., Schlom J., Travnicek M., Watson K. DNA-directed DNA polymerase activity in oncogenic RNA viruses. Nature. 1970 Sep 5;227(5262):1029–1031. doi: 10.1038/2271029a0. [DOI] [PubMed] [Google Scholar]
- Spiegelman S., Burny A., Das M. R., Keydar J., Schlom J., Trávnícek M., Watson K. Synthetic DNA-RNA hybrids and RNA-RNA duplexes as templates for the polymerases of the oncogenic RNA viruses. Nature. 1970 Oct 31;228(5270):430–432. doi: 10.1038/228430a0. [DOI] [PubMed] [Google Scholar]
- Spiegelman S., Watson K. F., Kacian D. L. Synthesis of DNA complements of natural RNAs: a general approach. Proc Natl Acad Sci U S A. 1971 Nov;68(11):2843–2845. doi: 10.1073/pnas.68.11.2843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Temin H. M., Mizutani S. RNA-dependent DNA polymerase in virions of Rous sarcoma virus. Nature. 1970 Jun 27;226(5252):1211–1213. doi: 10.1038/2261211a0. [DOI] [PubMed] [Google Scholar]
- Verma I. M., Meuth N. L., Bromfeld E., Manly K. F., Baltimore D. Covalently linked RNA-DNA molecule as initial product of RNA tumour virus DNA polymerase. Nat New Biol. 1971 Sep 29;233(39):131–134. doi: 10.1038/newbio233131a0. [DOI] [PubMed] [Google Scholar]