Abstract
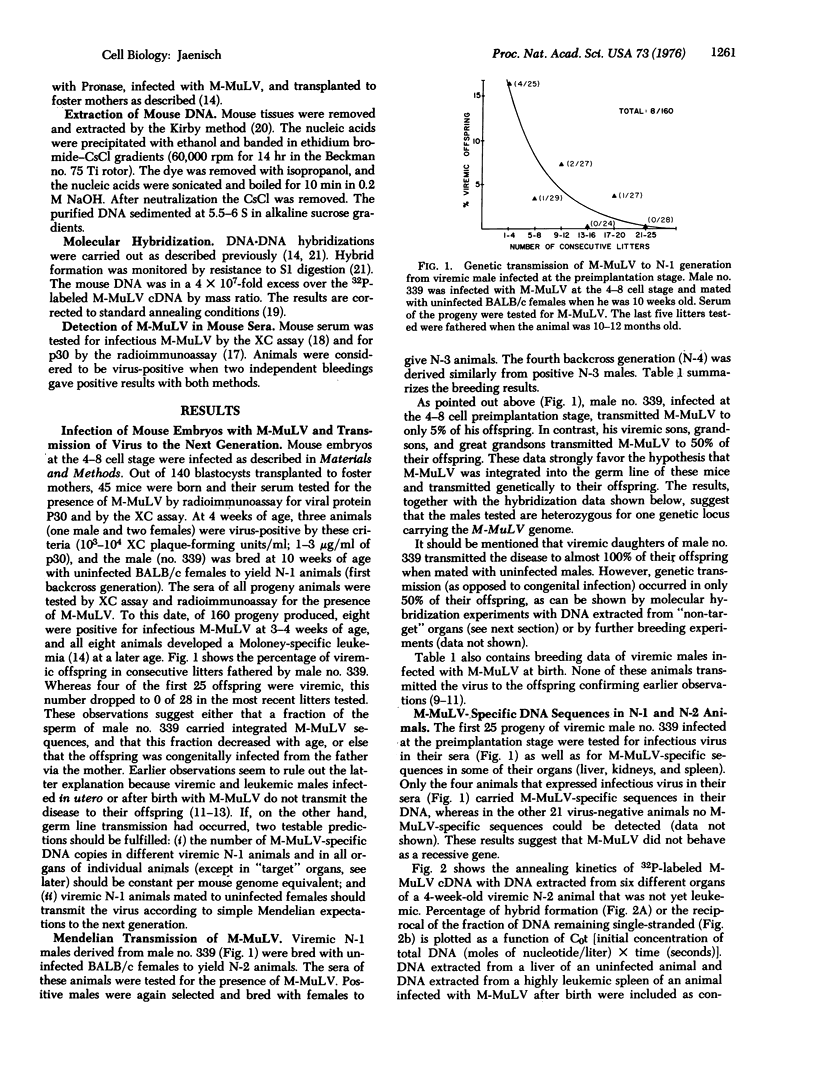
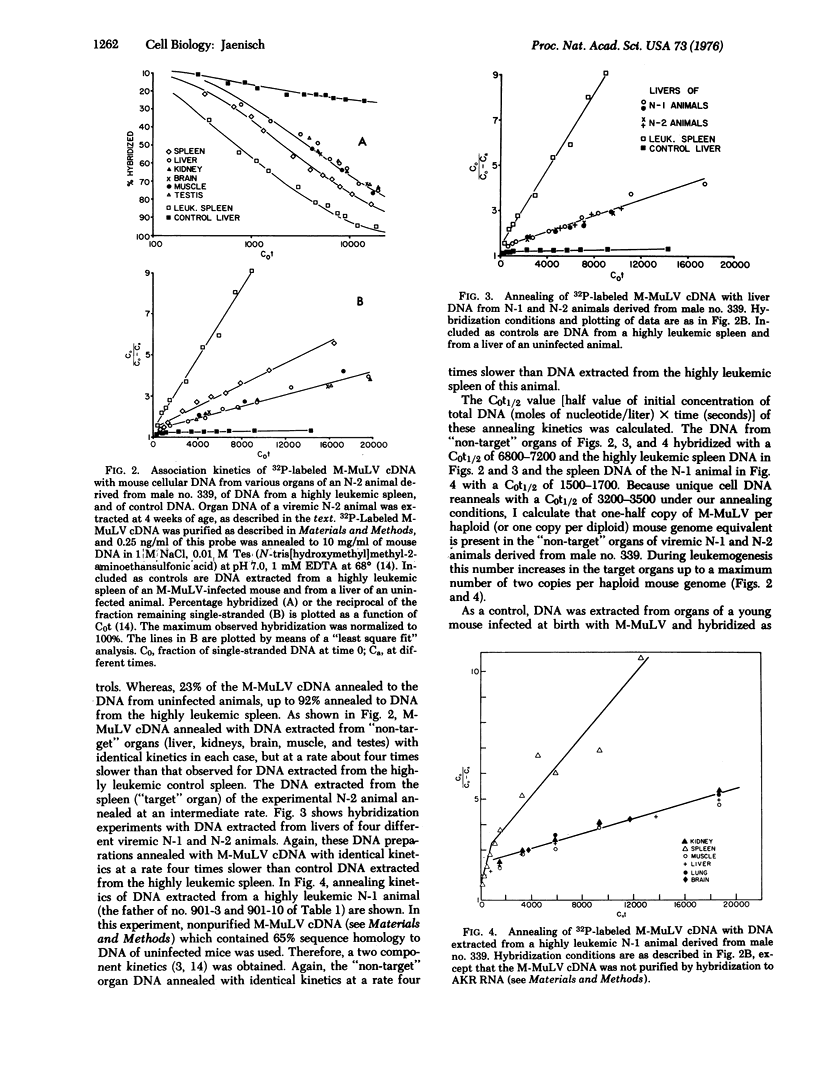
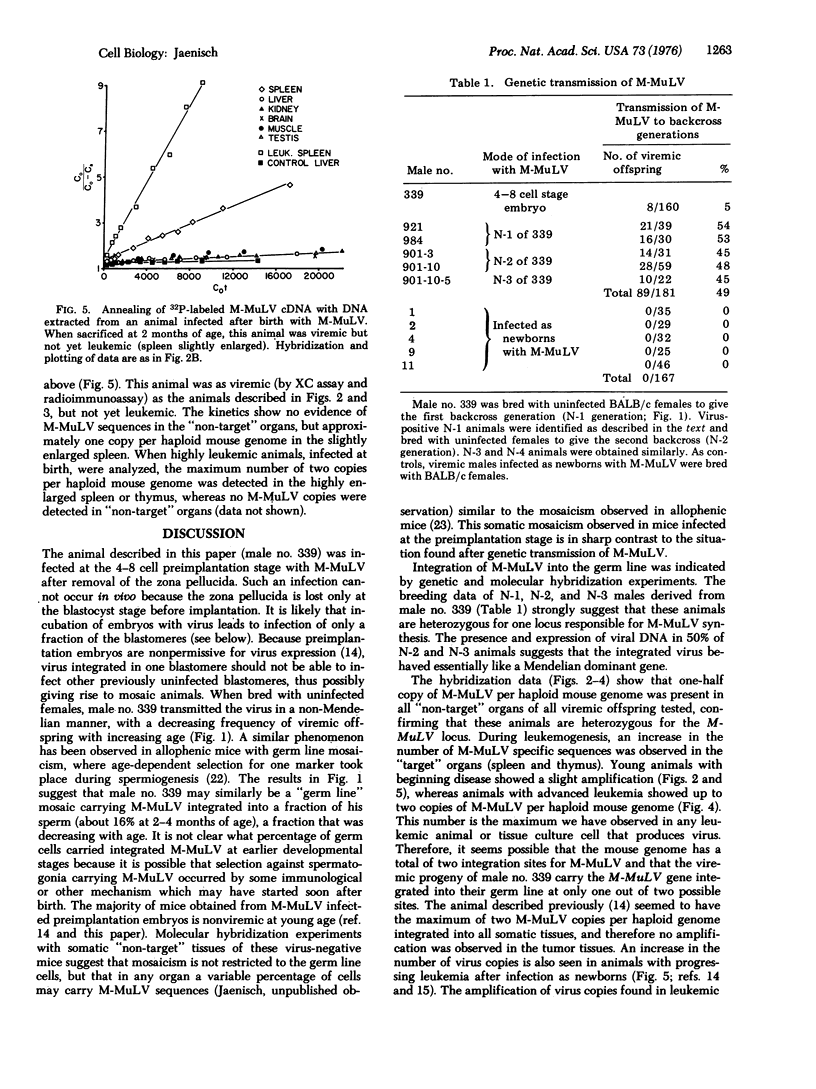
Mice were infected with the exogenous Moloney leukemia virus (M-MuLV) at two different stages of development. Either newborn mice (which can be considered as essentially fully differentiated animals) or preimplantation mouse embryos (at the 4-8 cell stage) were infected with M-MuLV. In both cases, animals that had developed an M-MuLV-induced leukemia were obtained. Two lines of evidence indicate that infection of preimplantation embryos, in contrast to infection of newborns, can lead to integration of the virus into the germ line. 1. Viremic males of the first backcross generation (N-1 generation) transmitted the virus to 50% of their offspring (N-2 generation) when mated with uninfected females. Likewise, a 50% transmission was observed from viremic N-2 and N-3 males to the next generations. 2. Molecular hybridization experiments revealed that viremic N-1 and N-2 animals carried one copy of M-MuLV per diploid mouse genome equivalent in all "non-target" organs tested. Together, both experiments indicate that the exogenous M-MuLV can be converted to an endogenous virus after infection of preimplantation embryos. The available evidence suggests that M-MuLV integrated into the germ line at one out of two possible integration sites. Thus, viremic backcross animals are heterozygous for a single Mendelian locus carrying the M-MuLV gene. During leukemogenesis an amplification of the M-MuLV from one copy to a maximum of four copies per diploid mouse genome equivalent takes place in the tumor tissues.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baluda M. A. Widespread presence, in chickens, of DNA complementary to the RNA genome of avian leukosis viruses. Proc Natl Acad Sci U S A. 1972 Mar;69(3):576–580. doi: 10.1073/pnas.69.3.576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benveniste R. E., Todaro G. J. Evolution of type C viral genes: preservation of ancestral murine type C viral sequences in pig cellular DNA. Proc Natl Acad Sci U S A. 1975 Oct;72(10):4090–4094. doi: 10.1073/pnas.72.10.4090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buffett R. F., Grace J. T., Jr, DiBerardino L. A., Mirand E. A. Vertical transmission of murine leukemia virus. Cancer Res. 1969 Mar;29(3):588–595. [PubMed] [Google Scholar]
- Chattopadhyay S. K., Lowy D. R., Teich N. M., Levine A. S., Rowe W. P. Qualitative and quantitative studies of AKR-type murine leukemia virus sequences in mouse DNA. Cold Spring Harb Symp Quant Biol. 1975;39(Pt 2):1085–1101. doi: 10.1101/sqb.1974.039.01.124. [DOI] [PubMed] [Google Scholar]
- Croker B. P., Jr, del Villano B. C., Jensen F. C., Lerner R. A., Dixon F. J. Immunopathogenicity and oncogenicity of murine leukemia viruses. I. Induction of immunologic disease and lymphoma in (BALB-c times NZB)F1 mice by Scripps leukemia virus. J Exp Med. 1974 Oct 1;140(4):1028–1048. doi: 10.1084/jem.140.4.1028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dofuku R., Biedler J. L., Spengler B. A., Old L. J. Trisomy of chromosome 15 in spontaneous leukemia of AKR mice. Proc Natl Acad Sci U S A. 1975 Apr;72(4):1515–1517. doi: 10.1073/pnas.72.4.1515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Essex M. Horizontally and vertically transmitted oncornaviruses of cats. Adv Cancer Res. 1975;21:175–248. doi: 10.1016/s0065-230x(08)60973-2. [DOI] [PubMed] [Google Scholar]
- Fan H., Baltimore D. RNA metabolism of murine leukemia virus: detection of virus-specific RNA sequences in infected and uninfected cells and identification of virus-specific messenger RNA. J Mol Biol. 1973 Oct 15;80(1):93–117. doi: 10.1016/0022-2836(73)90235-0. [DOI] [PubMed] [Google Scholar]
- Jaenisch R., Fan H., Croker B. Infection of preimplantation mouse embryos and of newborn mice with leukemia virus: tissue distribution of viral DNA and RNA and leukemogenesis in the adult animal. Proc Natl Acad Sci U S A. 1975 Oct;72(10):4008–4012. doi: 10.1073/pnas.72.10.4008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaenisch R., Mintz B. Simian virus 40 DNA sequences in DNA of healthy adult mice derived from preimplantation blastocysts injected with viral DNA. Proc Natl Acad Sci U S A. 1974 Apr;71(4):1250–1254. doi: 10.1073/pnas.71.4.1250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LAW L. W., MOLONEY J. B. Studies of congenital transmission of a leukemia virus in mice. Proc Soc Exp Biol Med. 1961 Dec;108:715–723. doi: 10.3181/00379727-108-27045. [DOI] [PubMed] [Google Scholar]
- Lieber M. M., Sherr C. J., Todaro G. J., Benveniste R. E., Callahan R., Coon H. G. Isolation from the asian mouse Mus caroli of an endogenous type C virus related to infectious primate type C viruses. Proc Natl Acad Sci U S A. 1975 Jun;72(6):2315–2319. doi: 10.1073/pnas.72.6.2315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mintz B. Clonal basis of mammalian differentiation. Symp Soc Exp Biol. 1971;25:345–370. [PubMed] [Google Scholar]
- Mintz B. Hermaphroditism, sex chromosomal mosaicism and germ cell selection in allophenic mice. J Anim Sci. 1968;27 (Suppl 1):51–60. [PubMed] [Google Scholar]
- Payne L. N., Chubb R. C. Studies on the nature and genetic control of an antigen in normal chick embryos which reacts in the COFAL test. J Gen Virol. 1968 Dec;3(3):379–391. doi: 10.1099/0022-1317-3-3-379. [DOI] [PubMed] [Google Scholar]
- RUBIN H., CORNELIUS A., FANSHIER L. The pattern of congenital transmission of an avian lekosis virus. Proc Natl Acad Sci U S A. 1961 Jul 15;47:1058–1069. doi: 10.1073/pnas.47.7.1058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowe W. P., Pugh W. E., Hartley J. W. Plaque assay techniques for murine leukemia viruses. Virology. 1970 Dec;42(4):1136–1139. doi: 10.1016/0042-6822(70)90362-4. [DOI] [PubMed] [Google Scholar]
- Rowe W. P. Studies of genetic transmission of murine leukemia virus by AKR mice. I. Crosses with Fv-1 n strains of mice. J Exp Med. 1972 Nov 1;136(5):1272–1285. doi: 10.1084/jem.136.5.1272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shoyab M., Baluda M. A. Acquisition of viral DNA sequences in target organs of chickens infected with avian myeloblastosis virus. J Virol. 1975 Oct;16(4):783–789. doi: 10.1128/jvi.16.4.783-789.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Todaro G. J., Benveniste R. E., Callahan R., Lieber M. M., Sherr C. J. Endogenous primate and feline type C viruses. Cold Spring Harb Symp Quant Biol. 1975;39(Pt 2):1159–1168. doi: 10.1101/sqb.1974.039.01.133. [DOI] [PubMed] [Google Scholar]


