Abstract
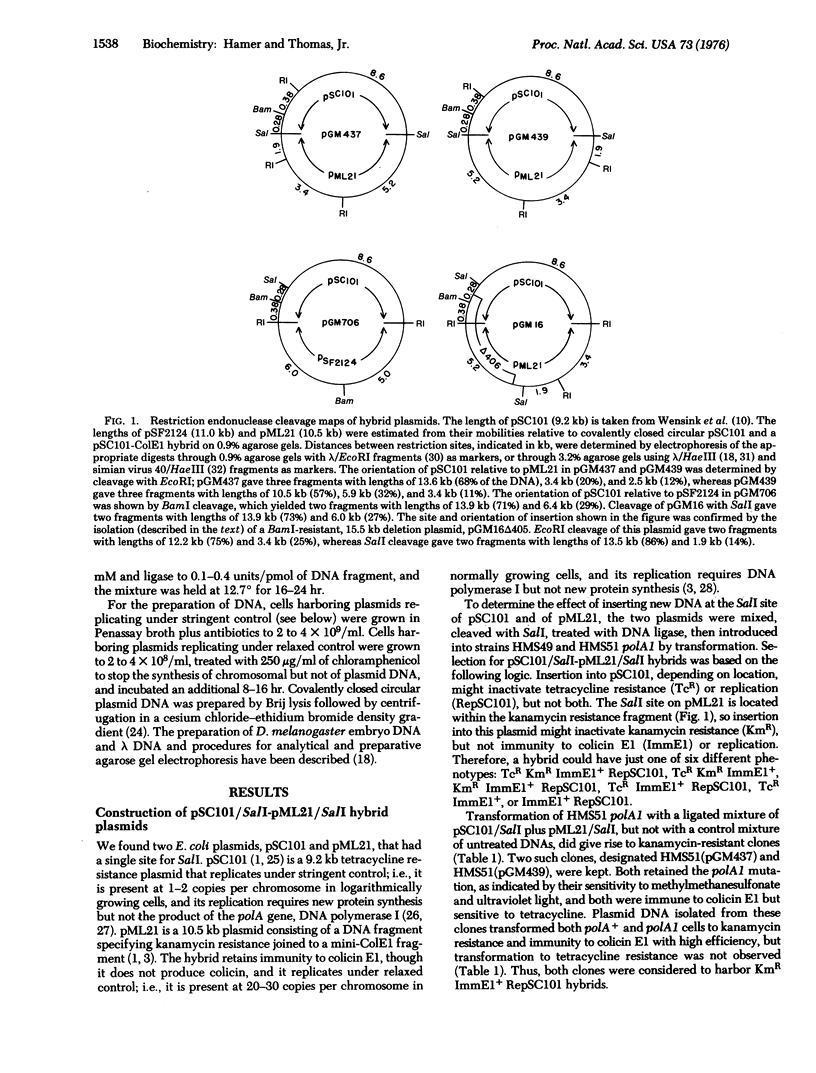
The highly specific restriction endonucleases Sa1I and BamI produce DNA fragments with complementary, cohesive termini that can be covalently joined by DNA ligase. The Escherichia coli kanamycin resistance factor pML21 has one SalI site, at which DNA can be inserted without interfering with the expression of drug resistance or replication of the plasmid. A more convenient cloning vehicle can be made with the tetracycline resistance factor pSC101, since insertion of DNA either at its single site for Sa1I or at that for BamI inactivates plasmid-specified drug resistance but not replication. To take advantage of this insertional inactivation, pSC101 was joined to a Co1E1-ampicillin resistance plasmid having no Sa1I site, and to a Co1E1-kanamycin resistance plasmid having no BamI site. Chimeras formed with the resulting hybrid vehicles can be identified simply by replica plating. These three vehicles, which all replicate under relaxed control, have been used to clone and amplify Drosophila melanogaster DNA fragments.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allet B., Solem R. Separation and analysis of promoter sites in bacteriophage lambda DNA by specific endonucleases. J Mol Biol. 1974 Jan 5;85(4):475–484. doi: 10.1016/0022-2836(74)90310-6. [DOI] [PubMed] [Google Scholar]
- Berg P., Baltimore D., Brenner S., Roblin R. O., Singer M. F. Summary statement of the Asilomar conference on recombinant DNA molecules. Proc Natl Acad Sci U S A. 1975 Jun;72(6):1981–1984. doi: 10.1073/pnas.72.6.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyer H. W., Roulland-Dussoix D. A complementation analysis of the restriction and modification of DNA in Escherichia coli. J Mol Biol. 1969 May 14;41(3):459–472. doi: 10.1016/0022-2836(69)90288-5. [DOI] [PubMed] [Google Scholar]
- Chang A. C., Cohen S. N. Genome construction between bacterial species in vitro: replication and expression of Staphylococcus plasmid genes in Escherichia coli. Proc Natl Acad Sci U S A. 1974 Apr;71(4):1030–1034. doi: 10.1073/pnas.71.4.1030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clewell D. B., Helinski D. R. Supercoiled circular DNA-protein complex in Escherichia coli: purification and induced conversion to an opern circular DNA form. Proc Natl Acad Sci U S A. 1969 Apr;62(4):1159–1166. doi: 10.1073/pnas.62.4.1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen S. N., Chang A. C., Boyer H. W., Helling R. B. Construction of biologically functional bacterial plasmids in vitro. Proc Natl Acad Sci U S A. 1973 Nov;70(11):3240–3244. doi: 10.1073/pnas.70.11.3240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen S. N., Chang A. C., Hsu L. Nonchromosomal antibiotic resistance in bacteria: genetic transformation of Escherichia coli by R-factor DNA. Proc Natl Acad Sci U S A. 1972 Aug;69(8):2110–2114. doi: 10.1073/pnas.69.8.2110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen S. N., Chang A. C. Recircularization and autonomous replication of a sheared R-factor DNA segment in Escherichia coli transformants. Proc Natl Acad Sci U S A. 1973 May;70(5):1293–1297. doi: 10.1073/pnas.70.5.1293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen S. N. The manipulation of genes. Sci Am. 1975 Jul;233(1):25–33. [PubMed] [Google Scholar]
- Glover D. M., White R. L., Finnegan D. J., Hogness D. S. Characterization of six cloned DNAs from Drosophila melanogaster, including one that contains the genes for rRNA. Cell. 1975 Jun;5(2):149–157. doi: 10.1016/0092-8674(75)90023-9. [DOI] [PubMed] [Google Scholar]
- Goebel W. Replication of the DNA of the colicinogenic factor E 1 (Col E 1 ) at the restrictive temperature in a DNA replication mutant thermosensitive for DNA polymerase. 3. Nat New Biol. 1972 May 17;237(72):67–70. doi: 10.1038/newbio237067a0. [DOI] [PubMed] [Google Scholar]
- Hedgpeth J., Goodman H. M., Boyer H. W. DNA nucleotide sequence restricted by the RI endonuclease. Proc Natl Acad Sci U S A. 1972 Nov;69(11):3448–3452. doi: 10.1073/pnas.69.11.3448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heffron F., Sublett R., Hedges R. W., Jacob A., Falkow S. Origin of the TEM-beta-lactamase gene found on plasmids. J Bacteriol. 1975 Apr;122(1):250–256. doi: 10.1128/jb.122.1.250-256.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hershfield V., Boyer H. W., Yanofsky C., Lovett M. A., Helinski D. R. Plasmid ColEl as a molecular vehicle for cloning and amplification of DNA. Proc Natl Acad Sci U S A. 1974 Sep;71(9):3455–3459. doi: 10.1073/pnas.71.9.3455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kedes L. H., Chang A. C., Houseman D., Cohen S. N. Isolation of histone genes from unfractionated sea urchin DNA by subculture cloning in E. coli. Nature. 1975 Jun 12;255(5509):533–538. doi: 10.1038/255533a0. [DOI] [PubMed] [Google Scholar]
- Kingsbury D. T., Helinski D. R. DNA polymerase as a requirement for the maintenance of the bacterial plasmid colicinogenic factor E1. Biochem Biophys Res Commun. 1970 Dec 24;41(6):1538–1544. doi: 10.1016/0006-291x(70)90562-0. [DOI] [PubMed] [Google Scholar]
- Lebowitz P., Siegel W., Sklar J. Hemophilus aegyptius restriction edonuclease cleavage map of the simian virus 40 genome and its colinear relation with the hemophilus influenzae cleavage map of SV40. J Mol Biol. 1974 Sep 5;88(1):105–123. doi: 10.1016/0022-2836(74)90297-6. [DOI] [PubMed] [Google Scholar]
- Mertz J. E., Davis R. W. Cleavage of DNA by R 1 restriction endonuclease generates cohesive ends. Proc Natl Acad Sci U S A. 1972 Nov;69(11):3370–3374. doi: 10.1073/pnas.69.11.3370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrow J. F., Cohen S. N., Chang A. C., Boyer H. W., Goodman H. M., Helling R. B. Replication and transcription of eukaryotic DNA in Escherichia coli. Proc Natl Acad Sci U S A. 1974 May;71(5):1743–1747. doi: 10.1073/pnas.71.5.1743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray N. E., Murray K. Manipulation of restriction targets in phage lambda to form receptor chromosomes for DNA fragments. Nature. 1974 Oct 11;251(5475):476–481. doi: 10.1038/251476a0. [DOI] [PubMed] [Google Scholar]
- Rambach A., Tiollais P. Bacteriophage lambda having EcoRI endonuclease sites only in the nonessential region of the genome. Proc Natl Acad Sci U S A. 1974 Oct;71(10):3927–3930. doi: 10.1073/pnas.71.10.3927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sgaramella V. Enzymatic oligomerization of bacteriophage P22 DNA and of linear Simian virus 40 DNA. Proc Natl Acad Sci U S A. 1972 Nov;69(11):3389–3393. doi: 10.1073/pnas.69.11.3389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka T., Weisblum B. Construction of a colicin E1-R factor composite plasmid in vitro: means for amplification of deoxyribonucleic acid. J Bacteriol. 1975 Jan;121(1):354–362. doi: 10.1128/jb.121.1.354-362.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka T., Weisblum B., Schnös M., Inman R. Construction and characterization of a chimeric plasmid composed of DNA Pfrom Escherichia coli and Drosophila melanogaster. Biochemistry. 1975 May 20;14(10):2064–2072. doi: 10.1021/bi00681a005. [DOI] [PubMed] [Google Scholar]
- Thomas M., Cameron J. R., Davis R. W. Viable molecular hybrids of bacteriophage lambda and eukaryotic DNA. Proc Natl Acad Sci U S A. 1974 Nov;71(11):4579–4583. doi: 10.1073/pnas.71.11.4579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas M., Davis R. W. Studies on the cleavage of bacteriophage lambda DNA with EcoRI Restriction endonuclease. J Mol Biol. 1975 Jan 25;91(3):315–328. doi: 10.1016/0022-2836(75)90383-6. [DOI] [PubMed] [Google Scholar]
- Timmis K., Cabello F., Cohen S. N. Utilization of two distinct modes of replication by a hybrid plasmid constructed in vitro from separate replicons. Proc Natl Acad Sci U S A. 1974 Nov;71(11):4556–4560. doi: 10.1073/pnas.71.11.4556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss B., Jacquemin-Sablon A., Live T. R., Fareed G. C., Richardson C. C. Enzymatic breakage and joining of deoxyribonucleic acid. VI. Further purification and properties of polynucleotide ligase from Escherichia coli infected with bacteriophage T4. J Biol Chem. 1968 Sep 10;243(17):4543–4555. [PubMed] [Google Scholar]
- Wensink P. C., Finnegan D. J., Donelson J. E., Hogness D. S. A system for mapping DNA sequences in the chromosomes of Drosophila melanogaster. Cell. 1974 Dec;3(4):315–325. doi: 10.1016/0092-8674(74)90045-2. [DOI] [PubMed] [Google Scholar]




