Abstract
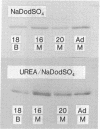
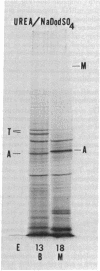
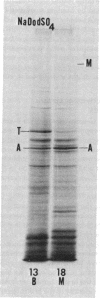
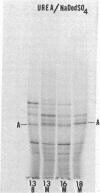
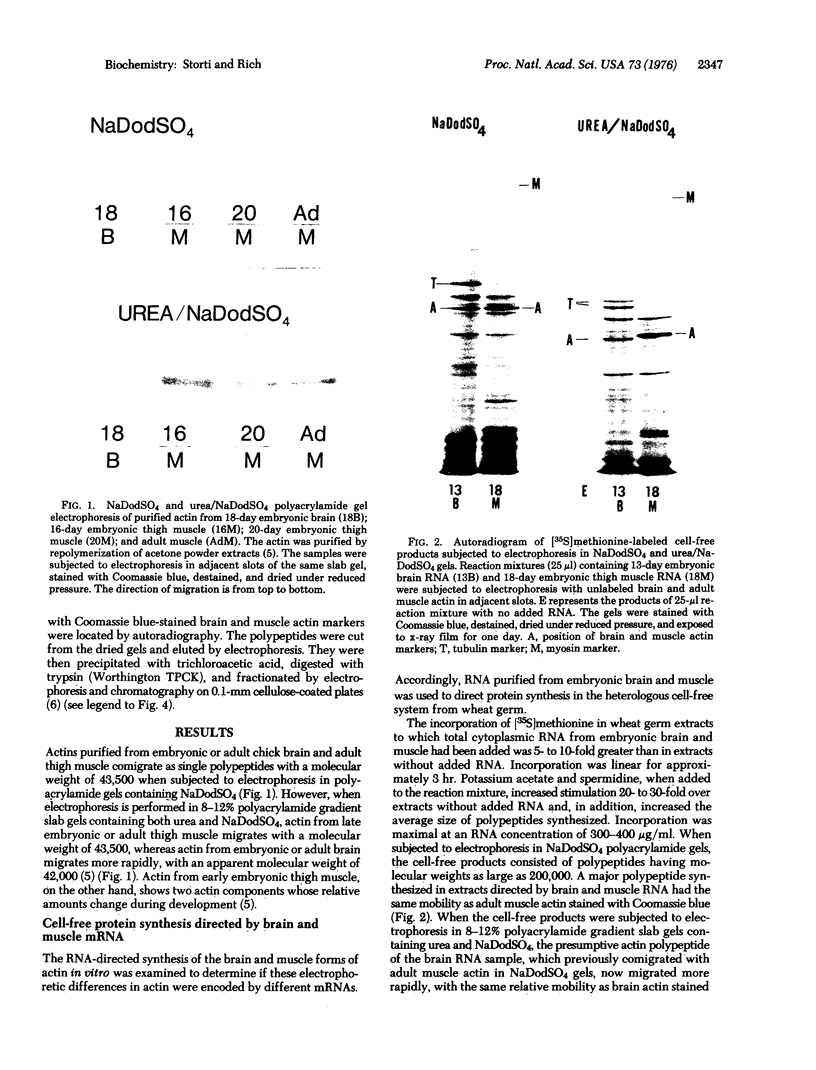
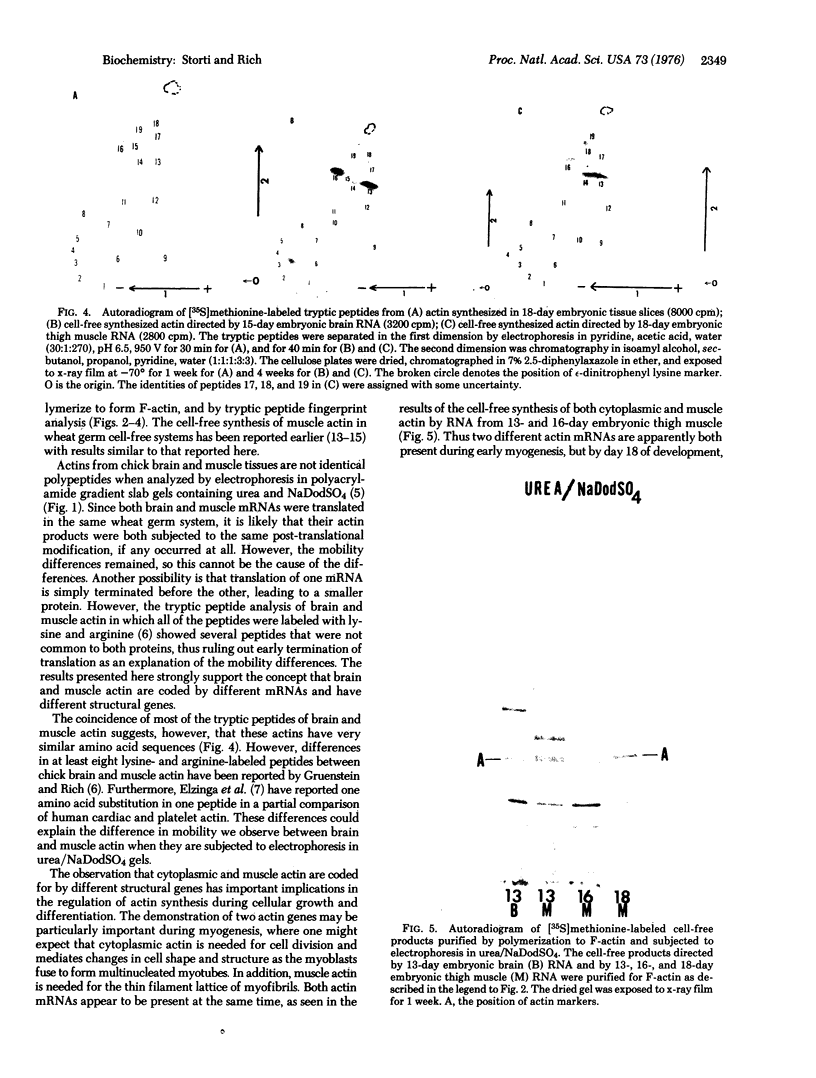
Actins isolated from embryonic chick brain and muscle differ in mobility when subjected to electrophoresis in gels containing urea and sodium dodecyl sulfate. Experiments were carried out to determine whether these actins are products of different structural genes and differ in primary amino acid sequence, or whether they are products of the same structural gene but are different because of post-translational modification. Messenger RNA from brain and muscle tissue was used to direct cell-free protein synthesis in wheat germ extracts. The synthesized actins were identified by conversion from globular to fibrous actin and by two-dimensional chromatographic analysis of tryptic peptides. The differences in electrophoretic mobility of brain compared to muscle actin were maintained in the cell-free protein synthetic products. Therefore, these mobility differences were not due to post-translational modification. It was concluded that brain and muscle actin are coded by different messenger RNAs and therefore arise from different structural genes. In addition, messenger RNA from 13- and 16-day embryonic thigh muscle directed the synthesis of both brain- and muscle-type actins, suggesting that muscle cell differentiation involves the regulation of at least two different actin genes.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bag J., Sarkar S. Cytoplasmic nonpolysomal messenger ribonucleoprotein containing actin messenger RNA in chicken embryonic muscles. Biochemistry. 1975 Aug 26;14(17):3800–3807. doi: 10.1021/bi00688a012. [DOI] [PubMed] [Google Scholar]
- Cohen I., Kaminski E., De Vries A. Actin-linked regulation of the human platelet contractile system. FEBS Lett. 1973 Aug 15;34(2):315–317. doi: 10.1016/0014-5793(73)80820-8. [DOI] [PubMed] [Google Scholar]
- Elzinga M., Maron B. J., Adelstein R. S. Human heart and platelet actins are products of different genes. Science. 1976 Jan 9;191(4222):94–95. doi: 10.1126/science.1246600. [DOI] [PubMed] [Google Scholar]
- Gozes I., Schmitt H., Littauer U. Z. Translation in vitro of rat brain messenger RNA coding for tubulin and actin. Proc Natl Acad Sci U S A. 1975 Feb;72(2):701–705. doi: 10.1073/pnas.72.2.701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruenstein E., Rich A. Non-identity of muscle and non-muscle actins. Biochem Biophys Res Commun. 1975 May 19;64(2):472–477. doi: 10.1016/0006-291x(75)90345-9. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lazarides E., Weber K. Actin antibody: the specific visualization of actin filaments in non-muscle cells. Proc Natl Acad Sci U S A. 1974 Jun;71(6):2268–2272. doi: 10.1073/pnas.71.6.2268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paterson B. M., Roberts B. E., Yaffe D. Determination of actin messenger RNA in cultures of differentiating embryonic chick skeletal muscle. Proc Natl Acad Sci U S A. 1974 Nov;71(11):4467–4471. doi: 10.1073/pnas.71.11.4467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollard T. D., Weihing R. R. Actin and myosin and cell movement. CRC Crit Rev Biochem. 1974 Jan;2(1):1–65. doi: 10.3109/10409237409105443. [DOI] [PubMed] [Google Scholar]
- Roberts B. E., Paterson B. M. Efficient translation of tobacco mosaic virus RNA and rabbit globin 9S RNA in a cell-free system from commercial wheat germ. Proc Natl Acad Sci U S A. 1973 Aug;70(8):2330–2334. doi: 10.1073/pnas.70.8.2330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger J. W. Presence of actin during chromosomal movement. Proc Natl Acad Sci U S A. 1975 Jun;72(6):2451–2455. doi: 10.1073/pnas.72.6.2451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer R. H., Penman S. Messenger RNA in HeLa cells: kinetics of formation and decay. J Mol Biol. 1973 Aug 5;78(2):321–334. doi: 10.1016/0022-2836(73)90119-8. [DOI] [PubMed] [Google Scholar]
- Spudich J. A., Watt S. The regulation of rabbit skeletal muscle contraction. I. Biochemical studies of the interaction of the tropomyosin-troponin complex with actin and the proteolytic fragments of myosin. J Biol Chem. 1971 Aug 10;246(15):4866–4871. [PubMed] [Google Scholar]