Abstract
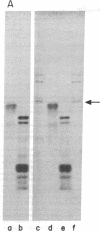
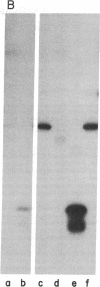
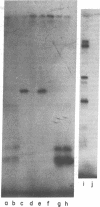
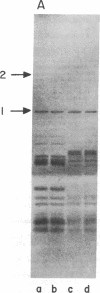
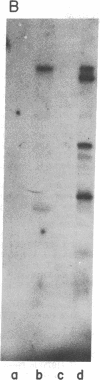
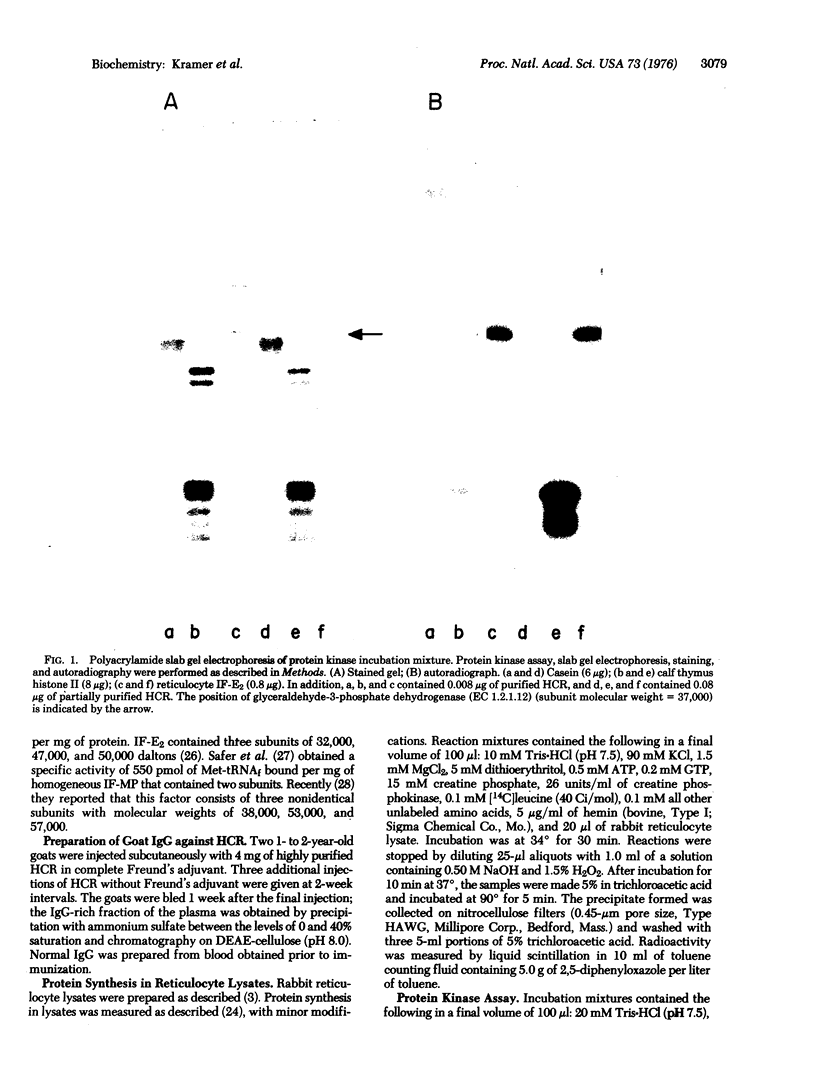
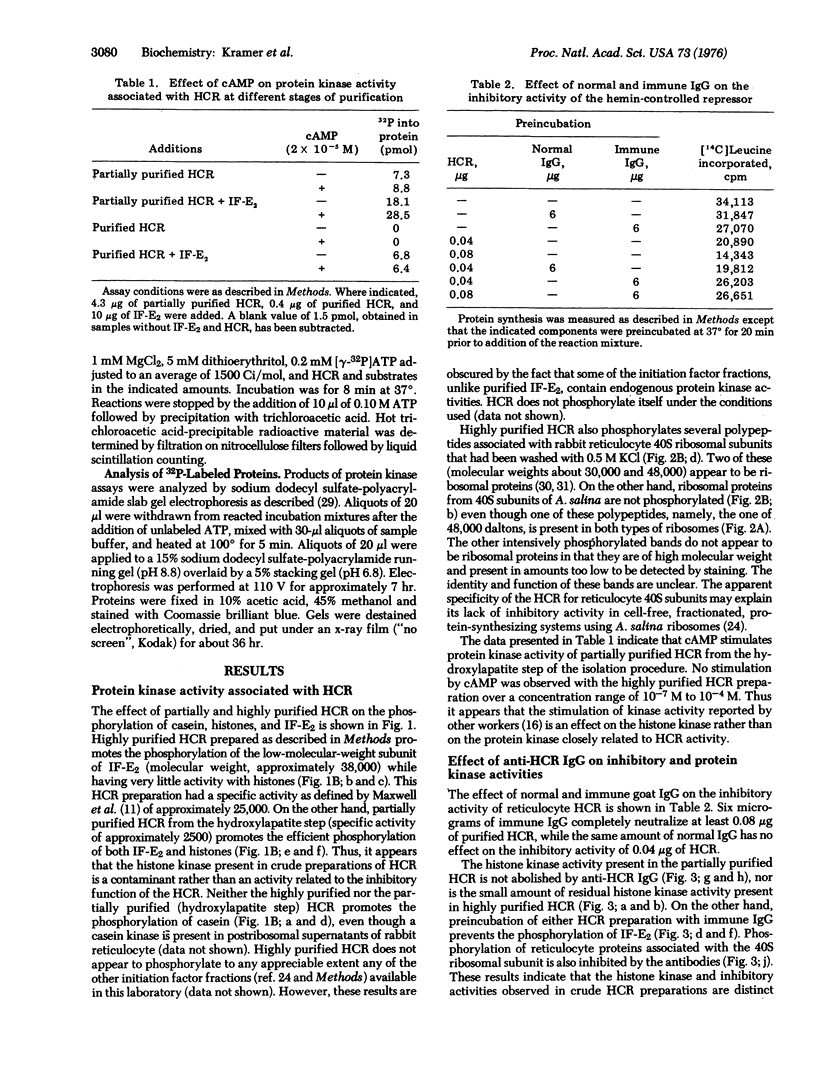
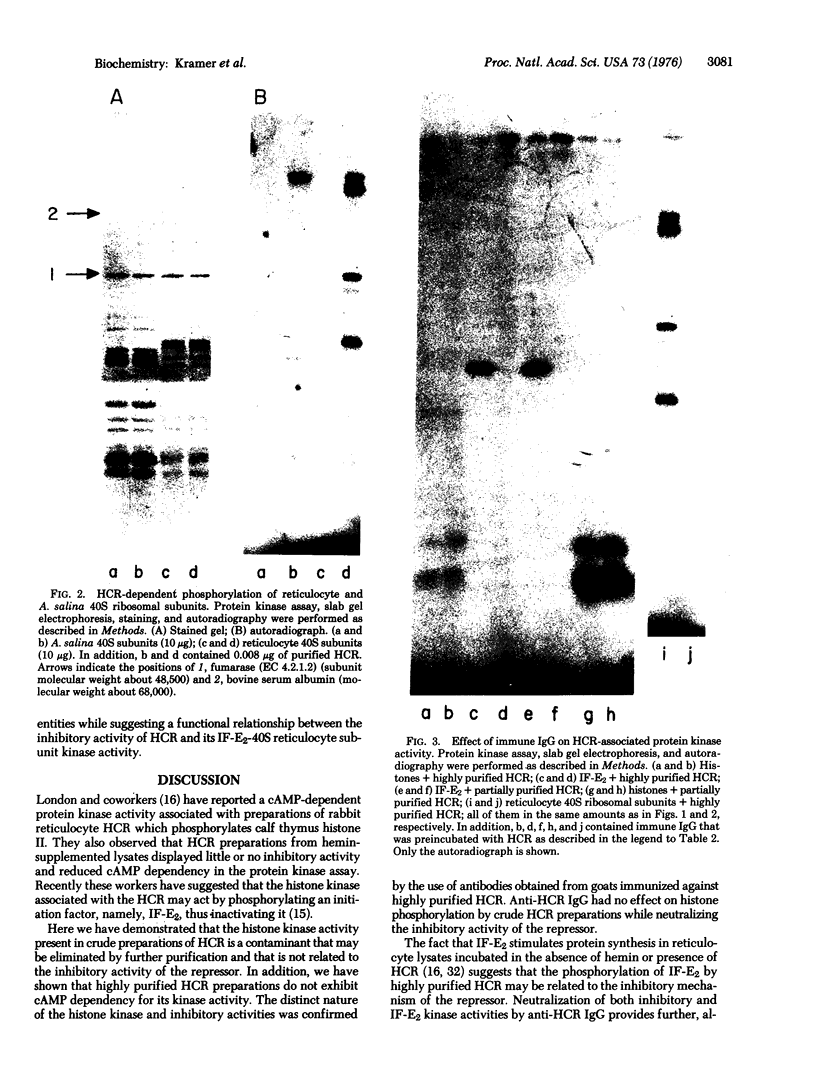
Highly purified preparations of hemin-controlled repressor of rabbit reticulocyte contain a 3':5'-cyclic AMP-indenpendent protein kinase activity that phosphorylates the low-molecular-weight (about 38,000) polypeptide chain of the initiation factor that forms a ternary complex with GTP and Met-tRNAf. These preparations also phosphorylate several polypeptide components of reticulocyte 40S ribosomal subunits. However, no significant levels of phosphorylation are observed when casein, histones, Artemia salina 40S ribosomal subunits, or other initiation factor fractions are used as substrates although high levels of phosphorylation are obtained with cruder preparations of the repressor. An antibody to these highly purified preparations of repressor has been obtained from the serum of immunized goats. Preincubation with immune goat IgG results in the neutralization of the inhibitory activity of the repressor, while normal IgG has no effect. Preincubation with immune IgG also abolishes the protein kinase activity responsible for the phosphorylation of the initiation factor and reticulocyte 40S subunits. Histone phosphorylation by crude repressor preparations, on the other hand, is unaffected by preincubation with immune IgG.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adamson S. D., Herbert E., Godchaux W. Factors affecting the rate of protein synthesis in lysate systems from reticulocytes. Arch Biochem Biophys. 1968 May;125(2):671–683. doi: 10.1016/0003-9861(68)90625-5. [DOI] [PubMed] [Google Scholar]
- BRUNS G. P., LONDON I. M. THE EFFECT OF HEMIN ON THE SYNTHESIS OF GLOBIN. Biochem Biophys Res Commun. 1965 Jan 18;18:236–242. doi: 10.1016/0006-291x(65)90746-1. [DOI] [PubMed] [Google Scholar]
- Balkow K., Hunt T., Jackson R. J. Control of protein synthesis in reticulocyte lysates: the effect of nucleotide triphosphates on formation of the translational repressor. Biochem Biophys Res Commun. 1975 Nov 3;67(1):366–375. doi: 10.1016/0006-291x(75)90325-3. [DOI] [PubMed] [Google Scholar]
- Balkow K., Mizuno S., Fisher J. M., Rabinovitz M. Hemin control of globin synthesis: effect of a translational repressor on Met-tRNAf binding to the small ribosomal subunit and its relation to the activity and alailability of an initiation factor. Biochim Biophys Acta. 1973 Oct 26;324(3):397–409. doi: 10.1016/0005-2787(73)90284-0. [DOI] [PubMed] [Google Scholar]
- Balkow K., Mizuno S., Rabinovitz M. Inhibition of an initiation codon function by hemin deficiency and the hemin-controlled translational repressor in the reticulocyte cell-free system. Biochem Biophys Res Commun. 1973 Sep 5;54(1):315–323. doi: 10.1016/0006-291x(73)90925-x. [DOI] [PubMed] [Google Scholar]
- Cimadevilla J. M., Kramer G., Pinphanichakarn P., Konecki D., Hardesty B. Inhibition of peptide chain initiation by a nonhemin-regulated translational repressor from Friend leukemia cells. Arch Biochem Biophys. 1975 Nov;171(1):145–153. doi: 10.1016/0003-9861(75)90017-x. [DOI] [PubMed] [Google Scholar]
- Ernst V., Levin D. H., Ranu R. S., London I. M. Control of protein synthesis in reticulocyte lysates: effects of 3':5'-cyclic AMP, ATP, and GTP on inhibitions induced by hemedeficiency, double-stranded RNA, and a reticulocyte translationa inhibitor. Proc Natl Acad Sci U S A. 1976 Apr;73(4):1112–1116. doi: 10.1073/pnas.73.4.1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falvey A. K., Staehelin T. Structure and function of mammalian ribosomes. I. Isolation and characterization of active liver ribosomal subunits. J Mol Biol. 1970 Oct 14;53(1):1–19. doi: 10.1016/0022-2836(70)90042-2. [DOI] [PubMed] [Google Scholar]
- Freienstein C., Blobel G. Nonribosomal proteins associated with eukaryotic native small ribosomal subunits. Proc Natl Acad Sci U S A. 1975 Sep;72(9):3392–3396. doi: 10.1073/pnas.72.9.3392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross M., Rabinovitz M. Partial purification of a translational repressor mediating hemin control of globin synthesis and implication of results on the site of inhibition. Biochem Biophys Res Commun. 1973 Feb 5;50(3):832–838. doi: 10.1016/0006-291x(73)91320-x. [DOI] [PubMed] [Google Scholar]
- Howard G. A., Adamson S. D., Herbert E. Studies on cessation of protein synthesis in a reticulocyte lysate cell-free system. Biochim Biophys Acta. 1970 Jul 16;213(1):237–240. doi: 10.1016/0005-2787(70)90028-6. [DOI] [PubMed] [Google Scholar]
- KRUH J., BORSOOK H. Hemoglobin synthesis in rabbit reticulocytes in vitro. J Biol Chem. 1956 Jun;220(2):905–915. [PubMed] [Google Scholar]
- Kramer G. A., Pinphanichakarn P., Konecki D., Hardesty B. A. Globin mRNA translation on Artemia salina ribosomes with components from Friend leukemia cells. Eur J Biochem. 1975 May 6;53(2):471–480. doi: 10.1111/j.1432-1033.1975.tb04088.x. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Legon S., Brayley A., Hunt T., Jackson R. J. The effect of cyclic AMP and related compounds on the control of protein synthesis in reticulocyte lysates. Biochem Biophys Res Commun. 1974 Feb 4;56(3):745–752. doi: 10.1016/0006-291x(74)90668-8. [DOI] [PubMed] [Google Scholar]
- Legon S., Jackson R. J., Hunt T. Control of protein synthesis in reticulocyte lysates by haemin. Nat New Biol. 1973 Jan 31;241(109):150–152. doi: 10.1038/newbio241150a0. [DOI] [PubMed] [Google Scholar]
- Levin D. H., Ranu R. S., Ernst V., Fifer M. A., London L. M. Association of a cyclic AMP-dependent protein kinase with a purified translational inhibitor isolated from hemin-deficient rabbit reticulocyte lysates. Proc Natl Acad Sci U S A. 1975 Dec;72(12):4849–4853. doi: 10.1073/pnas.72.12.4849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maxwell C. R., Kamper C. S., Rabinovitz M. Hemin control of globin synthesis: an assay for the inhibitor formed in the absence of hemin and some characteristics of its formation. J Mol Biol. 1971 May 28;58(1):317–327. doi: 10.1016/0022-2836(71)90249-x. [DOI] [PubMed] [Google Scholar]
- Miller R. L., Schweet R. Isolation of a protein fraction from reticulocyte ribosomes required for de novo synthesis of hemoglobin. Arch Biochem Biophys. 1968 May;125(2):632–646. doi: 10.1016/0003-9861(68)90622-x. [DOI] [PubMed] [Google Scholar]
- Obrig T., Irvin J., Culp W., Hardesty B. Inhibition of peptide initiation on reticulocyte ribosomes by edeine. Eur J Biochem. 1971 Jul 15;21(1):31–41. doi: 10.1111/j.1432-1033.1971.tb01436.x. [DOI] [PubMed] [Google Scholar]
- Safer B., Anderson W. F., Merrick W. C. Purification and physical properties of homogeneous initiation factor MP from rabbit reticulocytes. J Biol Chem. 1975 Dec 10;250(23):9067–9075. [PubMed] [Google Scholar]
- Schreier M. H., Staehelin T. Initiation of eukaryotic protein synthesis: (Met-tRNA f -40S ribosome) initiation complex catalysed by purified initiation factors in the absence of mRNA. Nat New Biol. 1973 Mar 14;242(115):35–38. doi: 10.1038/newbio242035a0. [DOI] [PubMed] [Google Scholar]
- Sundkvist I. C., Staehelin T. Structure and function of free 40 S ribosome subunits: Characterization of initiation factors. J Mol Biol. 1975 Dec 15;99(3):401–418. doi: 10.1016/s0022-2836(75)80135-5. [DOI] [PubMed] [Google Scholar]
- Zasloff M., Ochoa S. A supernatant factor involved in initiation complex formation with eukaryotic ribosomes. Proc Natl Acad Sci U S A. 1971 Dec;68(12):3059–3063. doi: 10.1073/pnas.68.12.3059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zucker W. V., Schulman H. M. Stimulation of globin-chain initiation by hemin in the reticulocyte cell-free system. Proc Natl Acad Sci U S A. 1968 Feb;59(2):582–589. doi: 10.1073/pnas.59.2.582. [DOI] [PMC free article] [PubMed] [Google Scholar]