Abstract
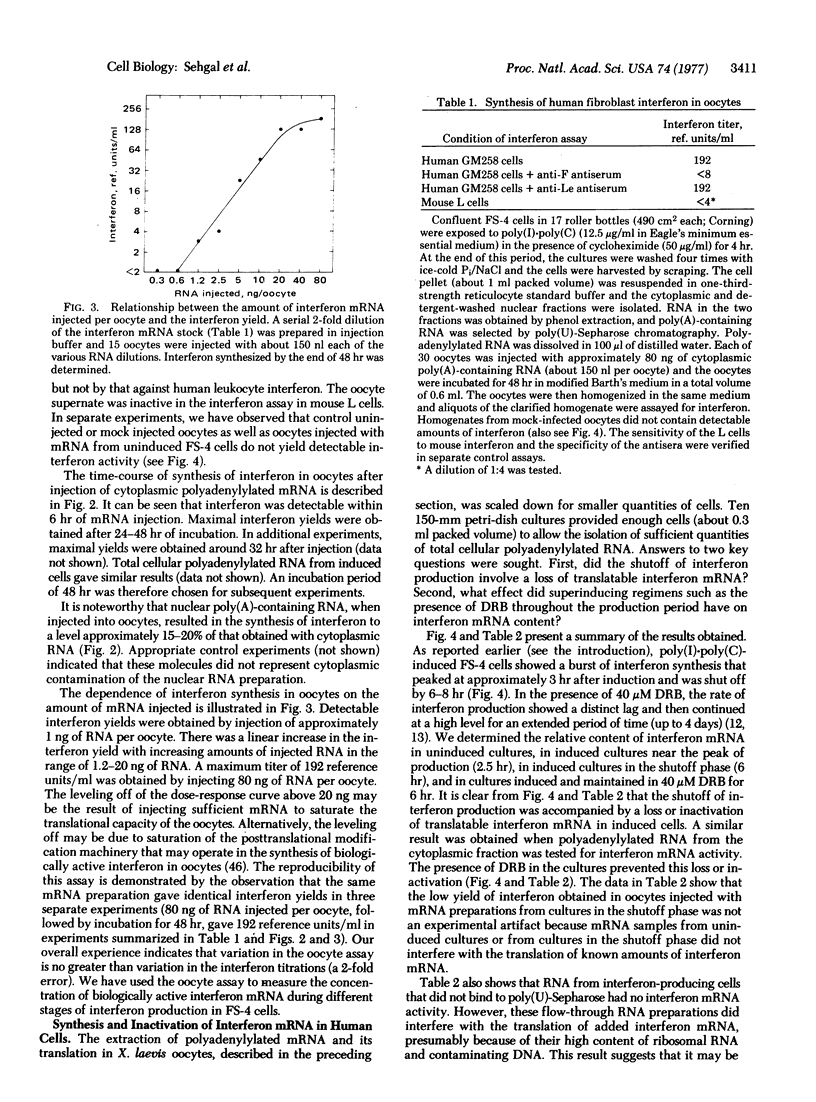
Translation of injected mRNA in oocytes of Xenopus laevis has been used as a highly sensitive and quantitative assay for interferon mRNA. Injection into oocytes of polyadenylylated RNA extracted from poly(I)·poly(C)-induced human diploid fibroblasts (FS-4) leads to the synthesis of biologically active human fibroblast interferon over a period of 24-32 hr. There is a linear relationship between the amount of mRNA injected and the interferon yield obtained over a range of 1-20 ng of injected RNA. Injection of 40-80 ng of mRNA into each of 15 oocytes, homogenized in 0.3 ml of incubation medium, gave a titer of 128-256 interferon reference units/ml of homogenate.
FS-4 cells at the peak of interferon production—i.e., approximately 2.5 hr after the beginning of induction with poly(I)·poly(C)—gave mRNA that yielded 24-48 interferon reference units/ml in the oocyte assay (30 ng of RNA injected per oocyte). An equivalent amount of mRNA from FS-4 cells in the shutoff phase, approximately 6 hr after induction, gave ≤4 interferon reference units/ml. In contrast, mRNA extracted from FS-4 cells that had been induced and maintained in the presence of 40 μM 5,6-dichloro-1-β-D-ribofuranosylbenzimidazole for 6 hr produced 64-128 interferon reference units/ml. Polyadenylylated RNA obtained from uninduced FS-4 cells did not lead to detectable interferon synthesis (<4 interferon reference units/ml). These data provide a direct verification of the hypothesis that the shutoff of interferon production in FS-4 cells involves a regulatory event leading to the posttranscriptional inactivation or degradation of interferon mRNA. Because the inactivating mechanism is sensitive to inhibition by 5,6-dichloro-1-β-D-ribofuranosylbenzimidazole, a selective inhibitor of nuclear heterogeneous RNA and mRNA synthesis, it is likely that synthesis of an RNA molecule is necessary for the shutoff of interferon production.
Keywords: human diploid fibroblast cells; poly(I)·poly(C); McAuslan-Tomkins translational repressor hypothesis; 5,6-dichloro-1-β-D-ribofuranosylbenzimidazole; Xenopus laevis oocytes
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adesnik M., Salditt M., Thomas W., Darnell J. E. Evidence that all messenger RNA molecules (except histone messenger RNA) contain Poly (A) sequences and that the Poly(A) has a nuclear function. J Mol Biol. 1972 Oct 28;71(1):21–30. doi: 10.1016/0022-2836(72)90397-x. [DOI] [PubMed] [Google Scholar]
- Armstrong J. A. Semi-micro, dye-binding assay for rabbit interferon. Appl Microbiol. 1971 Apr;21(4):723–725. doi: 10.1128/am.21.4.723-725.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blobel G., Dobberstein B. Transfer of proteins across membranes. I. Presence of proteolytically processed and unprocessed nascent immunoglobulin light chains on membrane-bound ribosomes of murine myeloma. J Cell Biol. 1975 Dec;67(3):835–851. doi: 10.1083/jcb.67.3.835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blobel G., Dobberstein B. Transfer of proteins across membranes. II. Reconstitution of functional rough microsomes from heterologous components. J Cell Biol. 1975 Dec;67(3):852–862. doi: 10.1083/jcb.67.3.852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bose S., Gurari-Rotman D., Ruegg U. T., Corley L., Anfinsen C. B. Apparent dispensability of the carbohydrate moiety of human interferon for antiviral activity. J Biol Chem. 1976 Mar 25;251(6):1659–1662. [PubMed] [Google Scholar]
- Cavalieri R. L., Havell E. A., Vilcek J., Pestka S. Synthesis of human interferon by Xenopus laevis oocytes: two structural genes for interferons in human cells. Proc Natl Acad Sci U S A. 1977 Aug;74(8):3287–3291. doi: 10.1073/pnas.74.8.3287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DULBECCO R., VOGT M. Plaque formation and isolation of pure lines with poliomyelitis viruses. J Exp Med. 1954 Feb;99(2):167–182. doi: 10.1084/jem.99.2.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derman E., Darnell J. E. Relationship of chain transcription to poly(A) addition and processing of hnRNA in HeLa cells. Cell. 1974 Nov;3(3):255–264. doi: 10.1016/0092-8674(74)90140-8. [DOI] [PubMed] [Google Scholar]
- Dobberstein B., Blobel G. Functional interaction of plant ribosomes with animal microsomal membranes. Biochem Biophys Res Commun. 1977 Feb 21;74(4):1675–1682. doi: 10.1016/0006-291x(77)90637-4. [DOI] [PubMed] [Google Scholar]
- EAGLE H. Amino acid metabolism in mammalian cell cultures. Science. 1959 Aug 21;130(3373):432–437. doi: 10.1126/science.130.3373.432. [DOI] [PubMed] [Google Scholar]
- Falcoff E., Havell E. A., Lewis J. A., Lande M. A., Falcoff R., Sabatini D. D., Vilcek J. Intracellular location of newly synthesized interferon in human FS-4 cells. Virology. 1976 Dec;75(2):384–393. doi: 10.1016/0042-6822(76)90037-4. [DOI] [PubMed] [Google Scholar]
- Field A. K., Tytell A. A., Lampson G. P., Hilleman M. R. Inducers of interferon and host resistance. II. Multistranded synthetic polynucleotide complexes. Proc Natl Acad Sci U S A. 1967 Sep;58(3):1004–1010. doi: 10.1073/pnas.58.3.1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GARREN L. D., HOWELL R. R., TOMKINS G. M., CROCCO R. M. A PARADOXICAL EFFECT OF ACTINOMYCIN D: THE MECHANISM OF REGULATION OF ENZYME SYNTHESIS BY HYDROCORTISONE. Proc Natl Acad Sci U S A. 1964 Oct;52:1121–1129. doi: 10.1073/pnas.52.4.1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Havell E. A., Berman B., Ogburn C. A., Berg K., Paucker K., Vilcek J. Two antigenically distinct species of human interferon. Proc Natl Acad Sci U S A. 1975 Jun;72(6):2185–2187. doi: 10.1073/pnas.72.6.2185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Havell E. A., Vilcek J., Falcoff E., Berman B. Suppression of human interferon production by inhibitors of glycosylation. Virology. 1975 Feb;63(2):475–483. doi: 10.1016/0042-6822(75)90320-7. [DOI] [PubMed] [Google Scholar]
- Havell E. A., Vilcek J. Production of high-titered interferon in cultures of human diploid cells. Antimicrob Agents Chemother. 1972 Dec;2(6):476–484. doi: 10.1128/aac.2.6.476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho M., Armstrong J. A. Interferon. Annu Rev Microbiol. 1975;29:131–161. doi: 10.1146/annurev.mi.29.100175.001023. [DOI] [PubMed] [Google Scholar]
- Killewich L., Schutz G., Feigelson P. Functional level of rat liver tryptophan 2,3-dixoygenase messenger RNA during superinduction of enzyme with actinomycin D. Proc Natl Acad Sci U S A. 1975 Nov;72(11):4285–4287. doi: 10.1073/pnas.72.11.4285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knight E., Jr Interferon: purification and initial characterization from human diploid cells. Proc Natl Acad Sci U S A. 1976 Feb;73(2):520–523. doi: 10.1073/pnas.73.2.520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohase M., Vilcek J. REgulation of human interferon production stimulated with poly(I)-poly(C): correlation between shutoff and hyporesponsiveness to reinduction. Virology. 1977 Jan;76(1):47–54. doi: 10.1016/0042-6822(77)90280-x. [DOI] [PubMed] [Google Scholar]
- MCAUSLAN B. R. THE INDUCTION AND REPRESSION OF THYMIDINE KINASE IN THE POXVIRUS-INFECTED HELA CELL. Virology. 1963 Nov;21:383–389. doi: 10.1016/0042-6822(63)90199-5. [DOI] [PubMed] [Google Scholar]
- Mullinix K. P., Wetekam W., Deeley R. G., Gordon J. I., Meyers M., Kent K. A., Goldberger R. F. Induction of vitellogenin synthesis by estrogen in avian liver: relationship between level of vitellogenin mRNA and vitellogenin synthesis. Proc Natl Acad Sci U S A. 1976 May;73(5):1442–1446. doi: 10.1073/pnas.73.5.1442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmiter R. D., Schimke R. T. Regulation of protein synthesis in chick oviduct. 3. Mechanism of ovalbumin "superinduction" by actinomycin D. J Biol Chem. 1973 Mar 10;248(5):1502–1512. [PubMed] [Google Scholar]
- Penman S., Vesco C., Penman M. Localization and kinetics of formation of nuclear heterodisperse RNA, cytoplasmic heterodisperse RNA and polyribosome-associated messenger RNA in HeLa cells. J Mol Biol. 1968 May 28;34(1):49–60. doi: 10.1016/0022-2836(68)90234-9. [DOI] [PubMed] [Google Scholar]
- Pestka S., McInnes J., Havell E. A., Vilcek J. Cell-free synthesis of human interferon. Proc Natl Acad Sci U S A. 1975 Oct;72(10):3898–3901. doi: 10.1073/pnas.72.10.3898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raj N. B., Pitha P. M. Relationship between interferon production and interferon messenger RNA synthesis in human fibroblasts. Proc Natl Acad Sci U S A. 1977 Apr;74(4):1483–1487. doi: 10.1073/pnas.74.4.1483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds F. H., Jr, Premkumar E., Pitha P. M. Interferon activity produced by translation of human interferon messenger RNA in cell-free ribosomal systems and in Xenopus oöcytes. Proc Natl Acad Sci U S A. 1975 Dec;72(12):4881–4885. doi: 10.1073/pnas.72.12.4881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sehgal P. B., Darnell J. E., Jr, Tamm I. The inhibition by DRB (5,6-dichloro-1-beta-D-ribofuranosylbenzimidazole) of hnRNA and mRNA production in HeLa cells. Cell. 1976 Nov;9(3):473–480. doi: 10.1016/0092-8674(76)90092-1. [DOI] [PubMed] [Google Scholar]
- Sehgal P. B., Derman E., Molloy G. R., Tamm I., Darnell J. E. 5,6-Dichloro-1-Beta-D-ribofuranosylbenzimidazole inhibits initiation of nuclear heterogeneous RNA chains in HeLa cells. Science. 1976 Oct 22;194(4263):431–433. doi: 10.1126/science.982026. [DOI] [PubMed] [Google Scholar]
- Sehgal P. B., Tamm I. An evaluation of messenger RNA competition in the shutoff of human interferon production. Proc Natl Acad Sci U S A. 1976 May;73(5):1621–1625. doi: 10.1073/pnas.73.5.1621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sehgal P. B., Tamm I., Vilcek J. Enhancement of human interferon production by neutral red and chloroquine: analysis of inhibition of protein degradation and macromolecular synthesis. J Exp Med. 1975 Nov 1;142(5):1283–1300. doi: 10.1084/jem.142.5.1283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sehgal P. B., Tamm I., Vilcek J. Human interferon production: superinduction by 5,6-dichloro-1-beta-D-ribofuranosylbenzimidazole. Science. 1975 Oct 17;190(4211):282–284. doi: 10.1126/science.1179208. [DOI] [PubMed] [Google Scholar]
- Sehgal P. B., Tamm I., Vilcek J. On the mechanism of enhancement of human interferon production by actinomycin D and cycloheximide. Virology. 1976 Mar;70(1):256–259. doi: 10.1016/0042-6822(76)90267-1. [DOI] [PubMed] [Google Scholar]
- Sehgal P. B., Tamm I., Vilcek J. Regulation of human interferon production. I. Superinduction by 5, 6-dichloro-1-beta-D-ribofuranosylbenzimidazole. Virology. 1976 Apr;70(2):532–541. doi: 10.1016/0042-6822(76)90294-4. [DOI] [PubMed] [Google Scholar]
- Sehgal P. B., Tamm I., Vilcek J. Regulation of human interferon production. II. Inhibition of interferon messenger RNA synthesis by 5, 6-dichloro-1-beta-D-ribofuranosylbenzimidazole. Virology. 1976 Apr;70(2):542–544. doi: 10.1016/0042-6822(76)90295-6. [DOI] [PubMed] [Google Scholar]
- Sussman P. M., Tushinski R. J., Bancroft F. C. Pregrowth hormone: product of the translation in vitro of messenger RNA coding for growth hormone. Proc Natl Acad Sci U S A. 1976 Jan;73(1):29–33. doi: 10.1073/pnas.73.1.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamm I., Hand R., Caliguiri L. A. Action of dichlorobenzimidazole riboside on RNA synthesis in L-929 and HeLa cells. J Cell Biol. 1976 May;69(2):229–240. doi: 10.1083/jcb.69.2.229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamm I., Sehgal P. B. A comparative study of the effects of certain halogenated benzimidazole ribosides on RNA synthesis, cell proliferation, and interferon production. J Exp Med. 1977 Feb 1;145(2):344–356. doi: 10.1084/jem.145.2.344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan Y. H., Armstrong J. A., Ke Y. H., Ho M. Regulation of cellular interferon production: enhancement by antimetabolites. Proc Natl Acad Sci U S A. 1970 Sep;67(1):464–471. doi: 10.1073/pnas.67.1.464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan Y. H. Chromosome 21 and the cell growth inhibitory effect of human interferon preparations. Nature. 1976 Mar 11;260(5547):141–143. doi: 10.1038/260141a0. [DOI] [PubMed] [Google Scholar]
- Thang M. N., Thang D. C., De Maeyer E., Montagnier L. Biosynthesis of mouse interferon by translation of its messenger RNA in a cell-free system. Proc Natl Acad Sci U S A. 1975 Oct;72(10):3975–3977. doi: 10.1073/pnas.72.10.3975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomkins G. M., Gelehrter T. D., Granner D., Martin D., Jr, Samuels H. H., Thompson E. B. Control of specific gene expression in higher organisms. Expression of mammalian genes may be controlled by repressors acting on the translation of messenger RNA. Science. 1969 Dec 19;166(3912):1474–1480. doi: 10.1126/science.166.3912.1474. [DOI] [PubMed] [Google Scholar]
- Tomkins G. M., Levinson B. B., Baxter J. D., Dethlefsen L. Further evidence for posttranscriptional control of inducible tyrosine aminotransferase synthesis in cultured hepatoma cells. Nat New Biol. 1972 Sep 6;239(88):9–14. doi: 10.1038/newbio239009a0. [DOI] [PubMed] [Google Scholar]
- Vilcek J., Havell E. A., Kohase M. Superinduction of interferon with metabolic inhibitors: possible mechanisms and practical applications. J Infect Dis. 1976 Jun;133 (Suppl):A22–A29. doi: 10.1093/infdis/133.supplement_2.a22. [DOI] [PubMed] [Google Scholar]
- Vilcek J., Havell E. A. Stabilization of interferon messenger RNA activity by treatment of cells with metabolic inhibitors and lowering of the incubation temperature. Proc Natl Acad Sci U S A. 1973 Dec;70(12):3909–3913. doi: 10.1073/pnas.70.12.3909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vilcek J., Rossman T. G., Varacalli F. Differential effects of actinomycin D and puromycin on the release of interferon induced by double stranded RNA. Nature. 1969 May 17;222(5194):682–683. doi: 10.1038/222682a0. [DOI] [PubMed] [Google Scholar]
- Villa-Komaroff L., Guttman N., Baltimore D., Lodishi H. F. Complete translation of poliovirus RNA in a eukaryotic cell-free system. Proc Natl Acad Sci U S A. 1975 Oct;72(10):4157–4161. doi: 10.1073/pnas.72.10.4157. [DOI] [PMC free article] [PubMed] [Google Scholar]


