Abstract
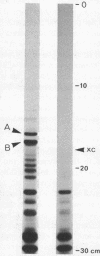
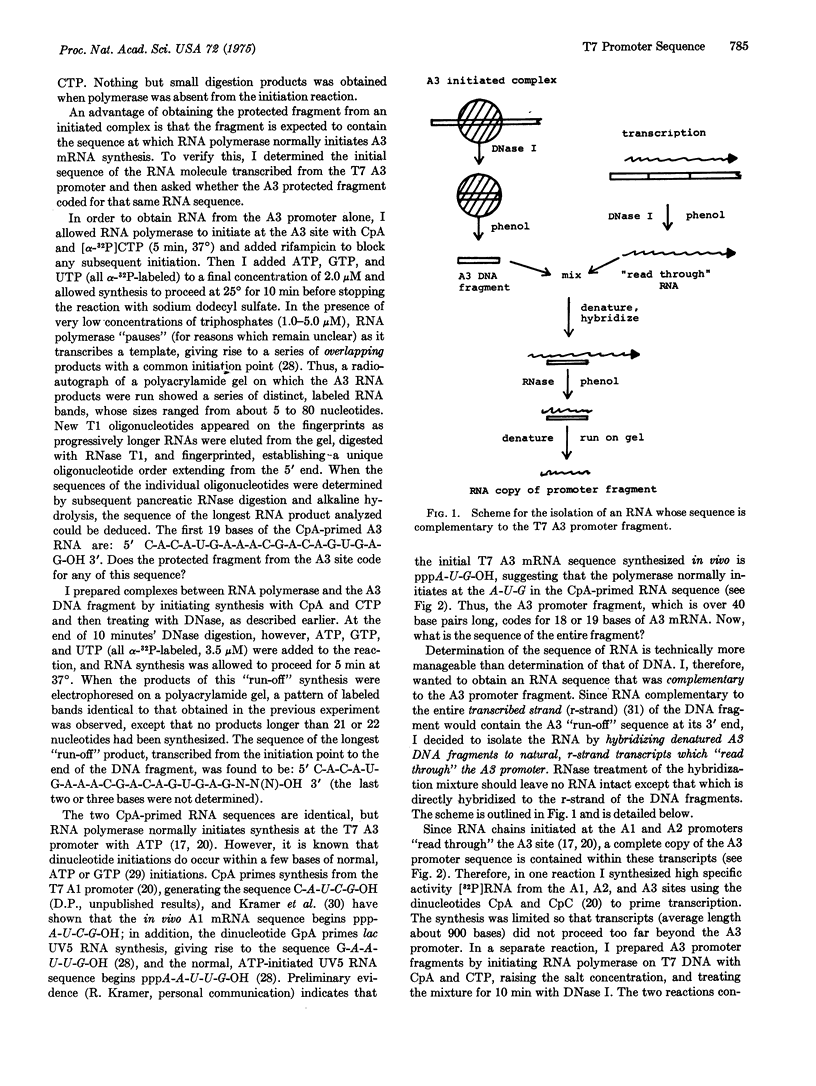
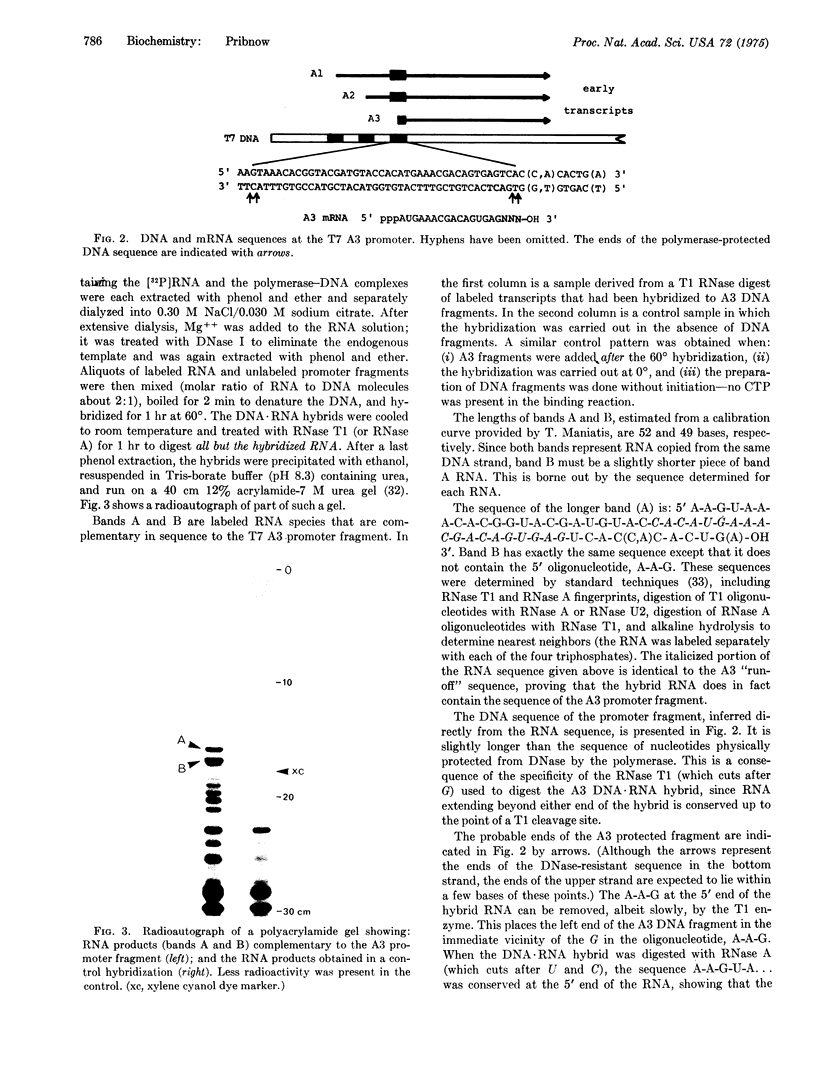
Escherichia coli RNA polymerase (EC 2.7.7.6), bound in a tight complex at an early T7 promoter, protects 41 to 43 base pairs of DNA from digestion by DNase. I. The protected DNA fragment contains both the binding site for RNA polymerase and the mRNA initiation point for the promoter. The sequence of the DNA fragment and the sequence of the mRNA that it codes for are presented here. A seven-base-pair sequence, apparently common to all promoters, is implicated in the formation of a tight binary complex with RNA polymerase.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arditti R. R., Scaife J. G., Beckwith J. R. The nature of mutants in the lac promoter region. J Mol Biol. 1968 Dec;38(3):421–426. doi: 10.1016/0022-2836(68)90396-3. [DOI] [PubMed] [Google Scholar]
- Beckwith J., Grodzicker T., Arditti R. Evidence for two sites in the lac promoter region. J Mol Biol. 1972 Aug 14;69(1):155–160. doi: 10.1016/0022-2836(72)90031-9. [DOI] [PubMed] [Google Scholar]
- Bordier C., Dubochet J. Electron microscopic localization of the binding sites of Escherichia coli RNA polymerase in the early promoter region of T7 DNA. Eur J Biochem. 1974 May 15;44(2):617–624. doi: 10.1111/j.1432-1033.1974.tb03519.x. [DOI] [PubMed] [Google Scholar]
- Chamberlin M. J., Ring J. Studies of the binding of Escherichia coli RNA polymerase to DNA. V. T7 RNA chain initiation by enzyme-DNA complexes. J Mol Biol. 1972 Sep 28;70(2):221–237. doi: 10.1016/0022-2836(72)90535-9. [DOI] [PubMed] [Google Scholar]
- Dhar R., Weissman S. M., Zain B. S., Pan J., Lewis A. M., Jr The nucleotide sequence preceding an RNA polymerase initiation site on SV40 DNA. Part 2. The sequence of the early strand transcript. Nucleic Acids Res. 1974 Apr;1(4):595–611. doi: 10.1093/nar/1.4.595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickson R. C., Abelson J., Barnes W. M., Reznikoff W. S. Genetic regulation: the Lac control region. Science. 1975 Jan 10;187(4171):27–35. doi: 10.1126/science.1088926. [DOI] [PubMed] [Google Scholar]
- Downey K. M., Jurmark B. S., So A. G. Determination of nucleotide sequences at promoter regions by the use of dinucleotides. Biochemistry. 1971 Dec 21;10(26):4970–4975. doi: 10.1021/bi00802a021. [DOI] [PubMed] [Google Scholar]
- Downey K. M., So A. G. Studies on the kinetics of ribonucleic acid chain initiation and elongation. Biochemistry. 1970 Jun 9;9(12):2520–2525. doi: 10.1021/bi00814a019. [DOI] [PubMed] [Google Scholar]
- Dunn J. J., Studier F. W. T7 early RNAs are generated by site-specific cleavages. Proc Natl Acad Sci U S A. 1973 May;70(5):1559–1563. doi: 10.1073/pnas.70.5.1559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eron L., Block R. Mechanism of initiation and repression of in vitro transcription of the lac operon of Escherichia coli. Proc Natl Acad Sci U S A. 1971 Aug;68(8):1828–1832. doi: 10.1073/pnas.68.8.1828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbert W., Maxam A. The nucleotide sequence of the lac operator. Proc Natl Acad Sci U S A. 1973 Dec;70(12):3581–3584. doi: 10.1073/pnas.70.12.3581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heyden B., Nüsslein C., Schaller H. Single RNA polymerase binding site isolated. Nat New Biol. 1972 Nov 1;240(96):9–12. doi: 10.1038/newbio240009a0. [DOI] [PubMed] [Google Scholar]
- Hinkle D. C., Chamberlin M. J. Studies of the binding of Escherichia coli RNA polymerase to DNA. I. The role of sigma subunit in site selection. J Mol Biol. 1972 Sep 28;70(2):157–185. doi: 10.1016/0022-2836(72)90531-1. [DOI] [PubMed] [Google Scholar]
- Hoffman D. J., Niyogi S. K. RNA initiation with dinucleoside monophosphates during transcription of bacteriophage T4 DNA with RNA polymerase of Escherichia coli. Proc Natl Acad Sci U S A. 1973 Feb;70(2):574–578. doi: 10.1073/pnas.70.2.574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopkins J. D. A new class of promoter mutations in the lactose operon of Escherichia coli. J Mol Biol. 1974 Aug 25;87(4):715–724. doi: 10.1016/0022-2836(74)90080-1. [DOI] [PubMed] [Google Scholar]
- Hyman R. W. Physical mapping of T7 messenger RNA. J Mol Biol. 1971 Oct 28;61(2):369–376. doi: 10.1016/0022-2836(71)90386-x. [DOI] [PubMed] [Google Scholar]
- Jones O. W., Berg P. Studies on the binding of RNA polymerase to polynucleotides. J Mol Biol. 1966 Dec 28;22(2):199–209. doi: 10.1016/0022-2836(66)90126-4. [DOI] [PubMed] [Google Scholar]
- Kramer R. A., Rosenberg M., Steitz J. A. Nucleotide sequences of the 5' and 3' termini of bacteriophage T7 early messenger RNAs synthesized in vivo: evidence for sequence specificity in RNA processing. J Mol Biol. 1974 Nov 15;89(4):767–776. doi: 10.1016/0022-2836(74)90051-5. [DOI] [PubMed] [Google Scholar]
- Le Talaer J. Y., Jeanteur P. Purification and base composition analysis of phage lambda early promoters. Proc Natl Acad Sci U S A. 1971 Dec;68(12):3211–3215. doi: 10.1073/pnas.68.12.3211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maitra U., Nakata Y., Hurwitz J. The role of deoxyribonucleic acid in ribonucleic acid synthesis. XIV. A study of the initiation of ribonucleic acid synthesis. J Biol Chem. 1967 Nov 10;242(21):4908–4918. [PubMed] [Google Scholar]
- Maizels N. M. The nucleotide sequence of the lactose messenger ribonucleic acid transcribed from the UV5 promoter mutant of Escherichia coli. Proc Natl Acad Sci U S A. 1973 Dec;70(12):3585–3589. doi: 10.1073/pnas.70.12.3585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mangel W. F., Chamberlin M. J. Studies of ribonucleic acid chain initiation by Escherichia coli ribonucleic acid polymerase bound to T7 deoxyribonucleic acid. 3. The effect of temperature on ribonucleic acid chain initiation and on the conformation of binary complexes. J Biol Chem. 1974 May 25;249(10):3007–3013. [PubMed] [Google Scholar]
- Mangel W. F., Chamberlin M. J. Studies of ribonucleic acid chain initiation by Escherichia coli ribonucleic acid polymerase bound to T7 deoxyribonucleic acid. II. The effect of alterations in ionic strength of chain initiation and on the conformation of binary complexes. J Biol Chem. 1974 May 25;249(10):3002–3006. [PubMed] [Google Scholar]
- Maniatis T., Ptashne M., Barrell B. G., Donelson J. Sequence of a repressor-binding site in the DNA of bacteriophage lamda. Nature. 1974 Aug 2;250(465):394–397. doi: 10.1038/250394a0. [DOI] [PubMed] [Google Scholar]
- Matsukage A., Murakami S., Kameyama T. The isolation and the characterization of DNA region bound to Escherichia coli RNA polymerase. Biochim Biophys Acta. 1969 Mar 18;179(1):145–157. doi: 10.1016/0005-2787(69)90130-0. [DOI] [PubMed] [Google Scholar]
- Maurer R., Maniatis T., Ptashne M. Promoters are in the operators in phage lambda. Nature. 1974 May 17;249(454):221–223. doi: 10.1038/249221a0. [DOI] [PubMed] [Google Scholar]
- Minkley E. G., Pribnow D. Transcription of the early region of bacteriophage T7: selective initiation with dinucleotides. J Mol Biol. 1973 Jun 25;77(2):255–277. doi: 10.1016/0022-2836(73)90335-5. [DOI] [PubMed] [Google Scholar]
- Okamoto T., Sugiura M., Takanami M. RNA polymerase binding sites of phage fd replicative form DNA. Nat New Biol. 1972 May 24;237(73):108–109. doi: 10.1038/newbio237108a0. [DOI] [PubMed] [Google Scholar]
- Richardson J. P. The binding of RNA polymerase to DNA. J Mol Biol. 1966 Oct 28;21(1):83–114. doi: 10.1016/0022-2836(66)90081-7. [DOI] [PubMed] [Google Scholar]
- Rüger W. Transcription of RNA polymerase binding sites isolated from T4 phage DNA. Biochim Biophys Acta. 1971 May 13;238(2):202–211. doi: 10.1016/0005-2787(71)90087-6. [DOI] [PubMed] [Google Scholar]
- Saucier J. M., Wang J. C. Angular alteration of the DNA helix by E. coli RNA polymerase. Nat New Biol. 1972 Oct 11;239(93):167–170. doi: 10.1038/newbio239167a0. [DOI] [PubMed] [Google Scholar]
- Schaller H., Gray C., Herrmann K. Nucleotide sequence of an RNA polymerase binding site from the DNA of bacteriophage fd. Proc Natl Acad Sci U S A. 1975 Feb;72(2):737–741. doi: 10.1073/pnas.72.2.737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sekiya T., Khorana H. G. Nucleotide sequence in the promoter region of the Escherichia coli tyrosine tRNA gene. Proc Natl Acad Sci U S A. 1974 Aug;71(8):2978–2982. doi: 10.1073/pnas.71.8.2978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sentenac A., Ruet A., Fromageot P. Excision de la region du DNA liee a la RNA polymerase in vitro. FEBS Lett. 1968 Nov;2(1):53–56. doi: 10.1016/0014-5793(68)80099-7. [DOI] [PubMed] [Google Scholar]
- Siegel R. B., Summers W. C. The process of infection with coliphage T7. 3. Control of phage-specific RNA synthesis in vivo by an early phage gene. J Mol Biol. 1970 Apr 14;49(1):115–123. doi: 10.1016/0022-2836(70)90380-3. [DOI] [PubMed] [Google Scholar]
- Silverstone A. E., Arditti R. R., Magasanik B. Catabolite-insensitive revertants of lac promoter mutants. Proc Natl Acad Sci U S A. 1970 Jul;66(3):773–779. doi: 10.1073/pnas.66.3.773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sippel A., Hartmann G. Mode of action of rafamycin on the RNA polymerase reaction. Biochim Biophys Acta. 1968 Mar 18;157(1):218–219. doi: 10.1016/0005-2787(68)90286-4. [DOI] [PubMed] [Google Scholar]
- Stead N. W., Jones O. W. Stability of RNA polymerase--DNA complexes. J Mol Biol. 1967 May 28;26(1):131–135. doi: 10.1016/0022-2836(67)90267-7. [DOI] [PubMed] [Google Scholar]