Abstract
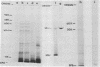
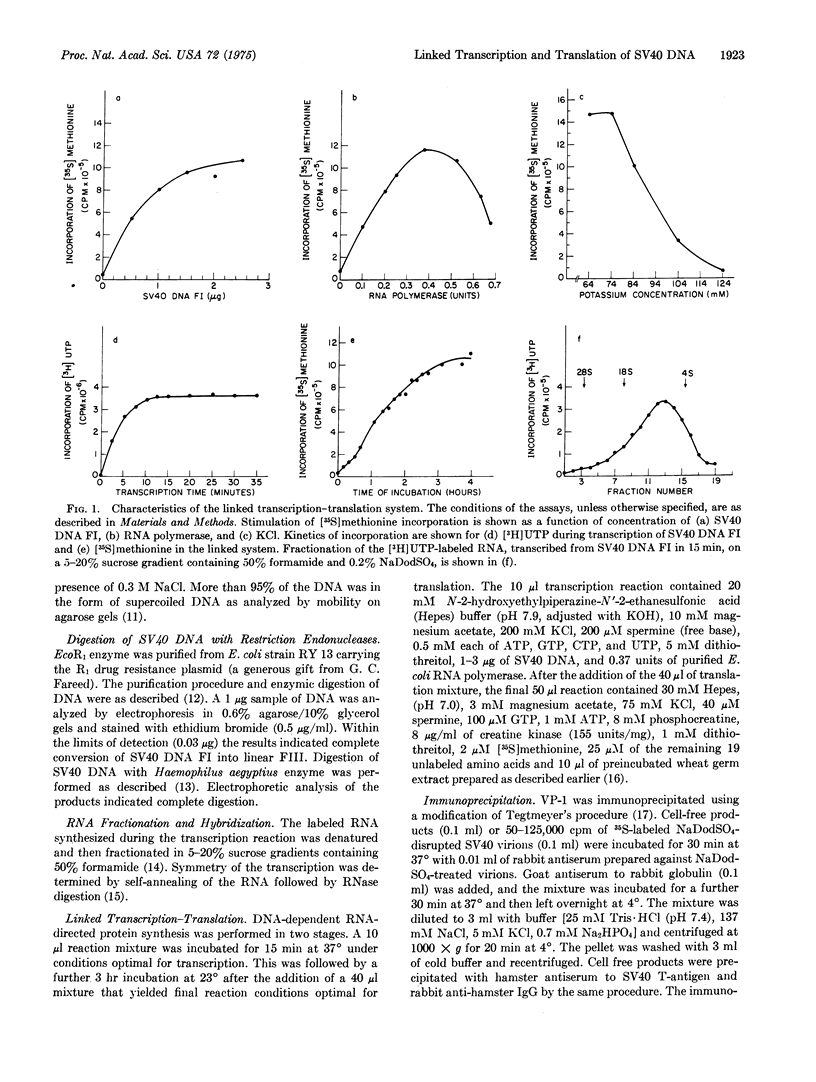
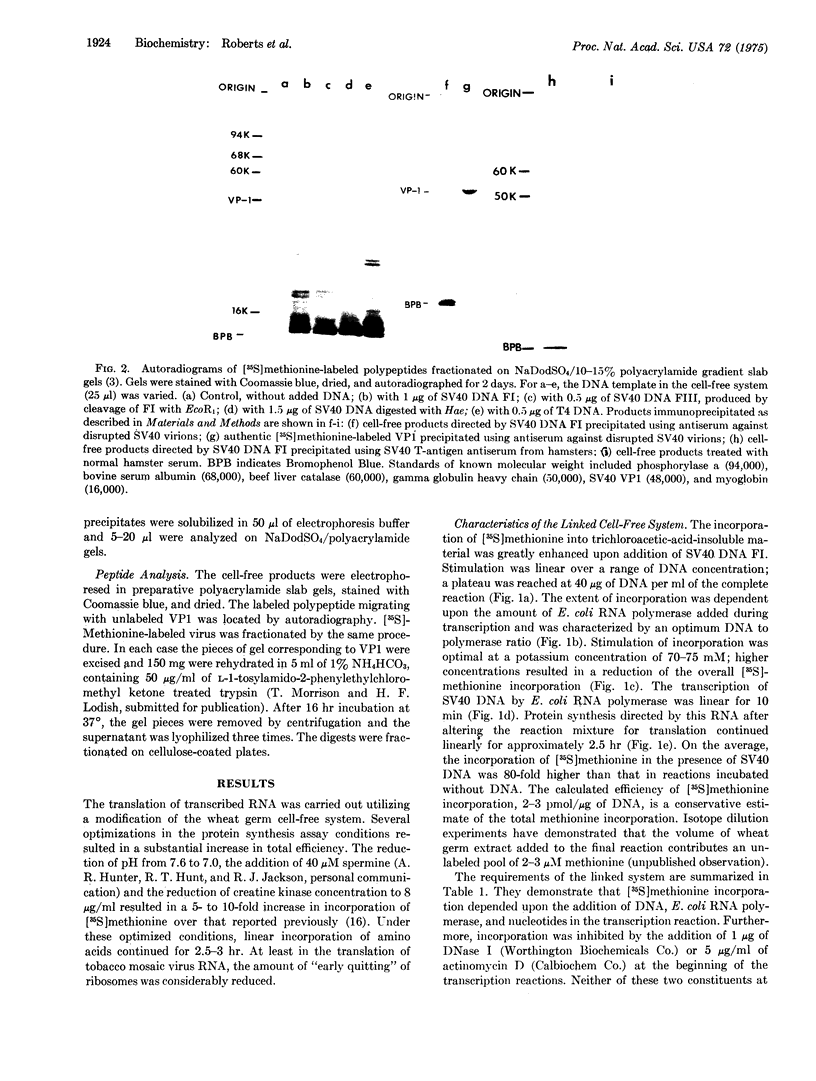
A linked cell-free system has been developed which is capable of transcribing and translating mamalian viral DNA, and its characteristics and requirements are outlined. In this system, simian virus 40 (SV40) DNA Form I (supercoiled) directed the synthesis of discrete polypeptides up to 85,000 daltons in size. One of these products was indistingusihable from authentic major virus capsid protein VPI, as judged by mobility on sodium dodecyl sulfate/polyacrylamide gels, antibody predipitation, and peptide analyses. The cell-free products larger than VPI comprised a number of polypeptides ranging in molecular weight from 50,000 to 85,000. These polypeptides demonstrated no immunological relationship whatsoever to the structural protein VPI. However, two of these products, along with one of approximately 25,000 dlatons, were precipitated with antiserum to SV40 tumor antigen. Linear SV40 DNA generated by the cleavage of Form I DNA with the restriction endonuclease EcoR1 was an efficient template in this system and also directed the synthesis of a polypeptide migrating with VPI on polyacrylamide gels. The potential of this system for defining a functional map of a DNA genome is discussed.
Full text
PDF
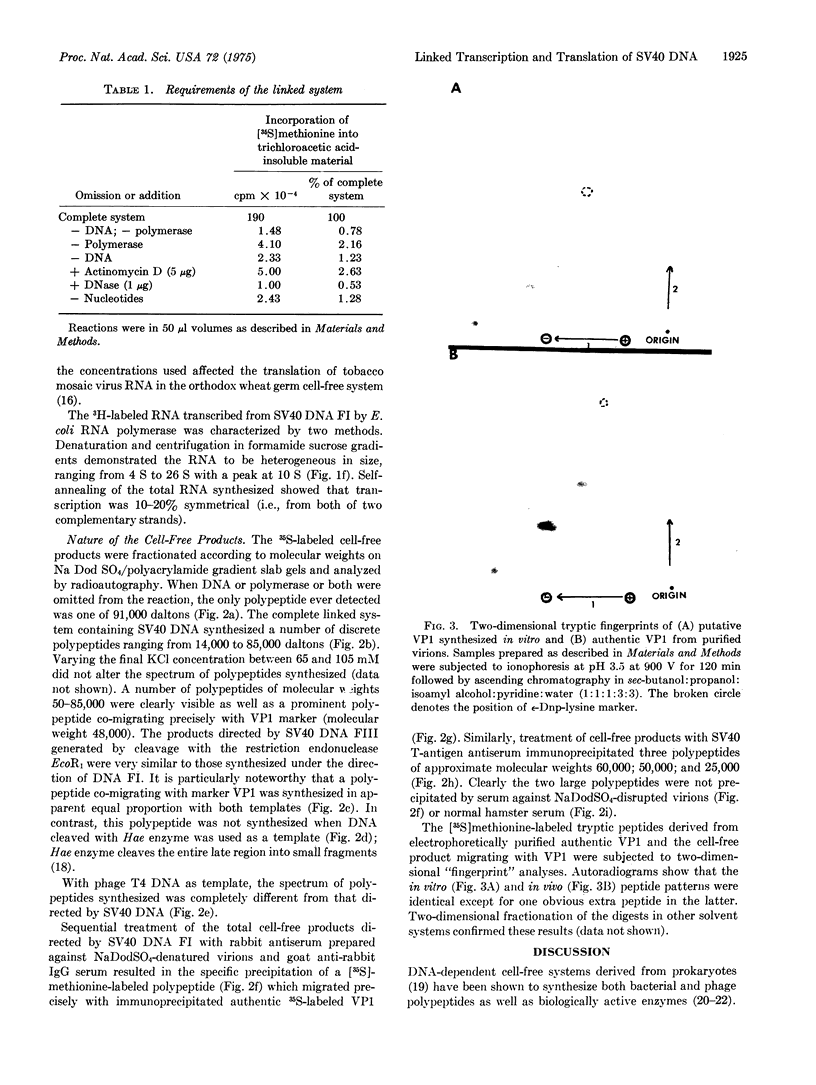

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson C. W., Lewis J. B., Atkins J. F., Gesteland R. F. Cell-free synthesis of adenovirus 2 proteins programmed by fractionated messenger RNA: a comparison of polypeptide products and messenger RNA lengths. Proc Natl Acad Sci U S A. 1974 Jul;71(7):2756–2760. doi: 10.1073/pnas.71.7.2756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryan R. N., Gelfand D. H., Hayashi M. Initiation of SV40 DNA-directed protein synthesis with N-formylmethionine in vitro. Nature. 1969 Dec 6;224(5223):1019–1021. doi: 10.1038/2241019b0. [DOI] [PubMed] [Google Scholar]
- Burgess R. R. A new method for the large scale purification of Escherichia coli deoxyribonucleic acid-dependent ribonucleic acid polymerase. J Biol Chem. 1969 Nov 25;244(22):6160–6167. [PubMed] [Google Scholar]
- Crawford L. V., Gesteland R. F. Synthesis of polyoma proteins in vitro. J Mol Biol. 1973 Mar 15;74(4):627–634. doi: 10.1016/0022-2836(73)90053-3. [DOI] [PubMed] [Google Scholar]
- Del Villano B. C., Defendi V. Characterization of the SV40 T antigen. Virology. 1973 Jan;51(1):34–46. doi: 10.1016/0042-6822(73)90363-2. [DOI] [PubMed] [Google Scholar]
- Estes M. K., Huang E. S., Pagano J. S. Structural polypeptides of simian virus 40. J Virol. 1971 May;7(5):635–641. doi: 10.1128/jvi.7.5.635-641.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gelfand D. H., Hayashi M. DNA-dependent RNA-directed protein synthesis in vitro, II. Synthesis of a phi-X-174 coat protein component. Proc Natl Acad Sci U S A. 1969 May;63(1):135–137. doi: 10.1073/pnas.63.1.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khoury G., Howley P., Nathans D., Martin M. Posttranscriptional selection of simian virus 40-specific RNA. J Virol. 1975 Feb;15(2):433–437. doi: 10.1128/jvi.15.2.433-437.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavi S., Winocour E. Acquisition of sequences homologous to host deoxyribonucleic acid by closed circular simian virus 40 deoxyribonucleic acid. J Virol. 1972 Feb;9(2):309–316. doi: 10.1128/jvi.9.2.309-316.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lebowitz P., Siegel W., Sklar J. Hemophilus aegyptius restriction edonuclease cleavage map of the simian virus 40 genome and its colinear relation with the hemophilus influenzae cleavage map of SV40. J Mol Biol. 1974 Sep 5;88(1):105–123. doi: 10.1016/0022-2836(74)90297-6. [DOI] [PubMed] [Google Scholar]
- Lodish H. F., Weinberg R., Ozer H. L. Translation of mRNA from simian virus 40-infected cells into simian virus 40 capsid protein by cell-free extracts. J Virol. 1974 Mar;13(3):590–595. doi: 10.1128/jvi.13.3.590-595.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Middleton J. H., Edgell M. H., Hutchison C. A., 3rd Specific fragments of phi X174 deoxyribonucleic acid produced by a restriction enzyme from Haemophilus aegyptius, endonuclease Z. J Virol. 1972 Jul;10(1):42–50. doi: 10.1128/jvi.10.1.42-50.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prives C. L., Aviv H., Paterson B. M., Roberts B. E., Rozenblatt S., Revel M., Winocour E. Cell-free translation of messenger RNA of simian virus 40: synthesis of the major capsid protein. Proc Natl Acad Sci U S A. 1974 Feb;71(2):302–306. doi: 10.1073/pnas.71.2.302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radloff R., Bauer W., Vinograd J. A dye-buoyant-density method for the detection and isolation of closed circular duplex DNA: the closed circular DNA in HeLa cells. Proc Natl Acad Sci U S A. 1967 May;57(5):1514–1521. doi: 10.1073/pnas.57.5.1514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts B. E., Paterson B. M. Efficient translation of tobacco mosaic virus RNA and rabbit globin 9S RNA in a cell-free system from commercial wheat germ. Proc Natl Acad Sci U S A. 1973 Aug;70(8):2330–2334. doi: 10.1073/pnas.70.8.2330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schweiger M., Gold L. M. DNA-dependent in vitro synthesis of bacteriophage enzymes. Cold Spring Harb Symp Quant Biol. 1969;34:763–766. doi: 10.1101/sqb.1969.034.01.086. [DOI] [PubMed] [Google Scholar]
- Tegtmeyer P. Altered patterns of protein synthesis in infection by SV40 mutants. Cold Spring Harb Symp Quant Biol. 1975;39(Pt 1):9–15. doi: 10.1101/sqb.1974.039.01.004. [DOI] [PubMed] [Google Scholar]
- Tegtmeyer P., Robb J. A., Widmer C., Ozer H. L. Altered protein metabolism in infection by the late tsB11 mutant of simian virus 40. J Virol. 1974 Oct;14(4):997–1007. doi: 10.1128/jvi.14.4.997-1007.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WOOD W. B., BERG P. The effect of enzymatically synthesized ribonucleic acid on amino acid incorporation by a soluble protein-ribosome system from Escherichia coli. Proc Natl Acad Sci U S A. 1962 Jan 15;48:94–104. doi: 10.1073/pnas.48.1.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westphal H. SV40 DNA strand selection by Escherichia coli RNA polymerase. J Mol Biol. 1970 Jun 14;50(2):407–420. doi: 10.1016/0022-2836(70)90201-9. [DOI] [PubMed] [Google Scholar]
- Zubay G., Chambers D. A. A DNA-directed cell-free system for beta-galactosidase synthesis; characterization of the de novo synthesized enzyme and some aspects of the regulation of synthesis. Cold Spring Harb Symp Quant Biol. 1969;34:753–761. doi: 10.1101/sqb.1969.034.01.085. [DOI] [PubMed] [Google Scholar]