Abstract
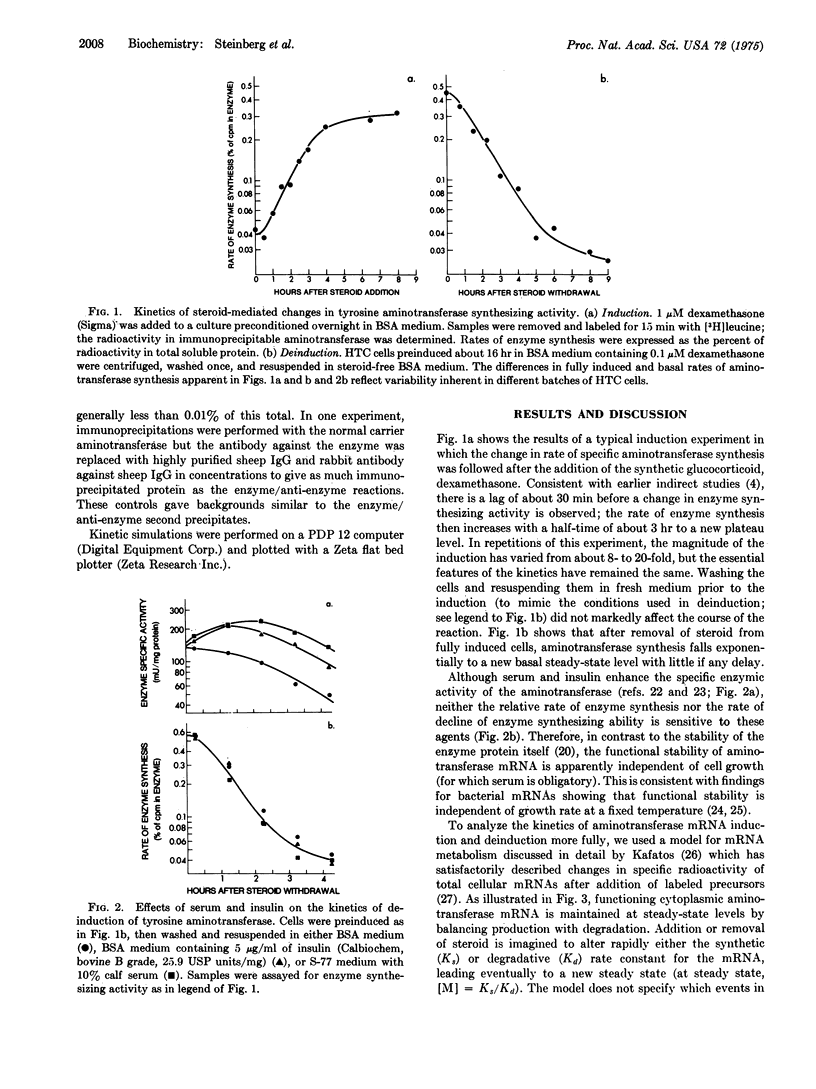
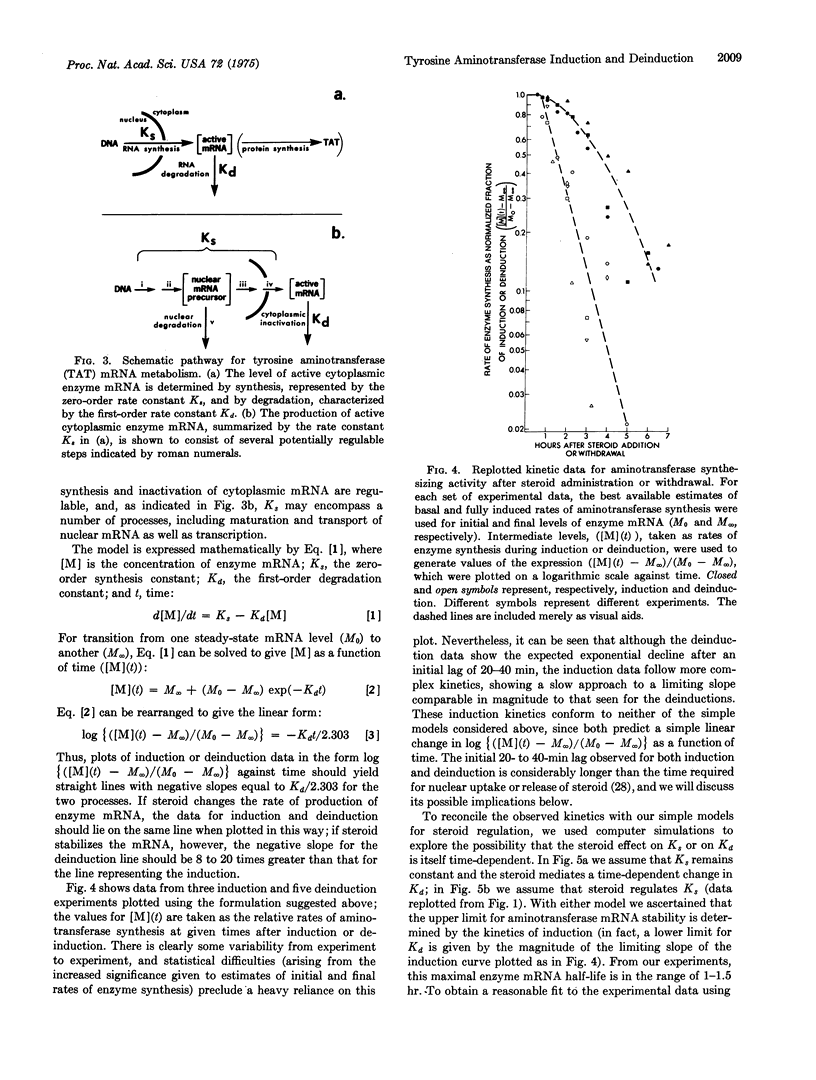
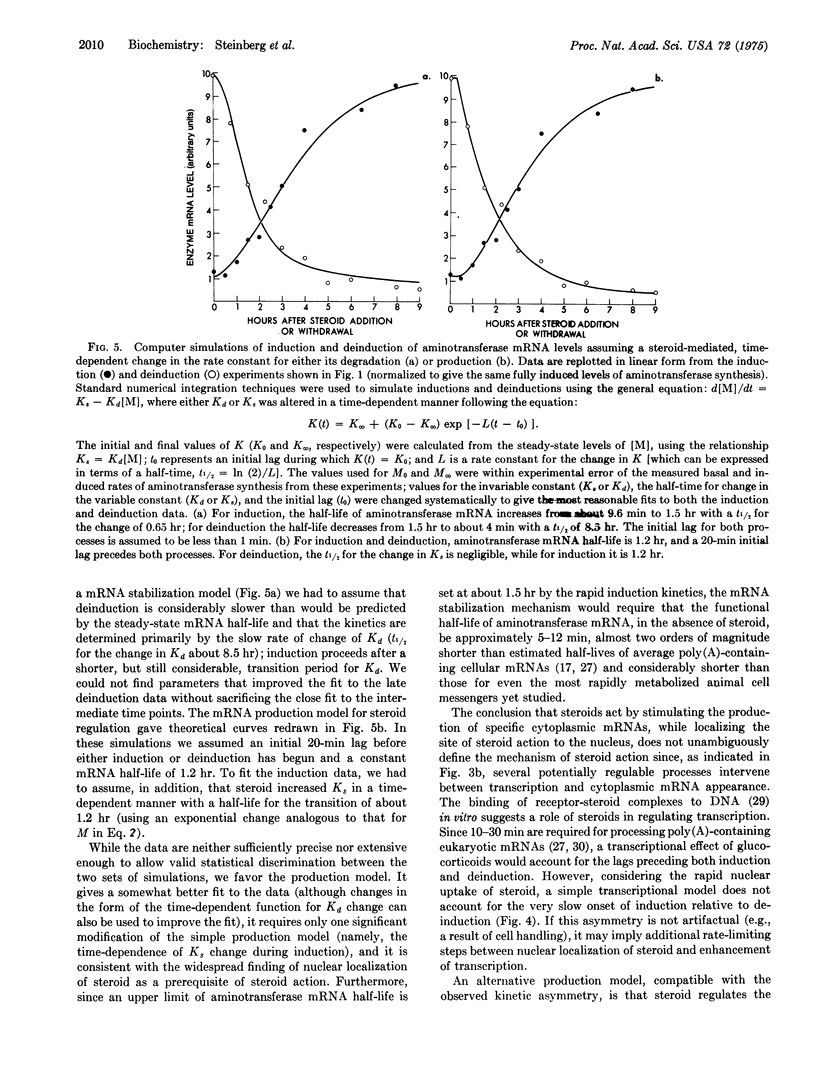
The specific rate of synthesis of tyrosine aminotransferase (EC 2.6.1.5; L-tyrosine:2-oxoglutarate aminotransferase) is used as a measure of the level of functional, cytoplasmic, tyrosine aminotransferase-specific mRNA in cultured rat hepatoma cells. An analysis of the kinetics of change in this rate after the addition or withdrawal of glucocorticosteroids sets an upper limit on the half-life of the enzyme-specific mRNA of 1-1.5 hr, whether or not steroid is present. The inactivation rate of the enzyme mRNA is independent of the growth condition of the cells, occuring equally rapidly in the presence or absence of serum or insulin, both of which induce tyrosine aminotransferase in these cells. The implications of these results for the mechanism of steroid induction are discussed.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adesnik M., Darnell J. E. Biogenesis and characterization of histone messenger RNA in HeLa cells. J Mol Biol. 1972 Jun 28;67(3):397–406. doi: 10.1016/0022-2836(72)90458-5. [DOI] [PubMed] [Google Scholar]
- Baxter J. D., Rousseau G. G., Benson M. C., Garcea R. L., Ito J., Tomkins G. M. Role of DNA and specific cytoplasmic receptors in glucocorticoid action. Proc Natl Acad Sci U S A. 1972 Jul;69(7):1892–1896. doi: 10.1073/pnas.69.7.1892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baxter J. D., Tomkins G. M. The relationship between glucocorticoid binding and tyrosine aminotransferase induction in hepatoma tissue culture cells. Proc Natl Acad Sci U S A. 1970 Mar;65(3):709–715. doi: 10.1073/pnas.65.3.709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck J. P., Beck G., Wong K. Y., Tomkins G. M. Synthesis of inducible tyrosine aminotransferase in a cell-free extract from cultured hepatoma cells. Proc Natl Acad Sci U S A. 1972 Dec;69(12):3615–3619. doi: 10.1073/pnas.69.12.3615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berlin C. M., Schimke R. T. Influence of turnover rates on the responses of enzymes to cortisone. Mol Pharmacol. 1965 Sep;1(2):149–156. [PubMed] [Google Scholar]
- Bushnell D. E., Becker J. E., Potter V. R. The role of messenger RNA in tyrosine aminotransferase superinduction: effects of camptothecin on hepatoma cells in culture. Biochem Biophys Res Commun. 1974 Feb 4;56(3):815–821. doi: 10.1016/0006-291x(74)90678-0. [DOI] [PubMed] [Google Scholar]
- Chan L., Means A. R., O'Malley B. W. Rates of induction of specific translatable messenger RNAs for ovalbumin and avidin by steroid hormones. Proc Natl Acad Sci U S A. 1973 Jun;70(6):1870–1874. doi: 10.1073/pnas.70.6.1870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gelehrter T. D., Tomkins G. M. Control of tyrosine aminotransferase synthesis in tissue culture by a factor in serum. Proc Natl Acad Sci U S A. 1969 Oct;64(2):723–730. doi: 10.1073/pnas.64.2.723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gelehrter T. D., Tomkins G. M. Posttranscriptional control of tyrosine aminotransferase synthesis by insulin. Proc Natl Acad Sci U S A. 1970 Jun;66(2):390–397. doi: 10.1073/pnas.66.2.390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Granner D. K., Hayashi S., Thompson E. B., Tomkins G. M. Stimulation of tyrosine aminotransferase synthesis by dexamethasone phosphate in cell culture. J Mol Biol. 1968 Jul 28;35(2):291–301. doi: 10.1016/s0022-2836(68)80025-7. [DOI] [PubMed] [Google Scholar]
- Granner D. K., Thompson E. B., Tomkins G. M. Dexamethasone phosphate-induced synthesis of tyrosine aminotransferase in hepatoma tissue culture cells. Studies of the early phases of induction and of the steroid requirement for maintanance of the induced rate of synthesis. J Biol Chem. 1970 Mar 25;245(6):1472–1478. [PubMed] [Google Scholar]
- Greenberg J. R. High stability of messenger RNA in growing cultured cells. Nature. 1972 Nov 10;240(5376):102–104. doi: 10.1038/240102a0. [DOI] [PubMed] [Google Scholar]
- Hershko A., Tomkins G. M. Studies on the degradation of tyrosine aminotransferase in hepatoma cells in culture. Influence of the composition of the medium and adenosine triphosphate dependence. J Biol Chem. 1971 Feb 10;246(3):710–714. [PubMed] [Google Scholar]
- Kennell D., Bicknell I. Decay of messenger ribonucleic acid from the lactose operon of Escherichia coli as a function of growth temperature. J Mol Biol. 1973 Feb 15;74(1):21–31. doi: 10.1016/0022-2836(73)90351-3. [DOI] [PubMed] [Google Scholar]
- Kenney F. T., Lee K. L., Stiles C. D., Fritz J. E. Further evidence against post-transcriptional control of inducible tyrosine aminotransferase synthesis in cultured hepatoma cells. Nat New Biol. 1973 Dec 19;246(155):208–210. doi: 10.1038/newbio246208a0. [DOI] [PubMed] [Google Scholar]
- Kenney F., Lee K. L., Reel J. R., Hoel D. G. Regulation of tyrosine alpha-ketoglutarate transaminase in rat liver. IX. Studies of the mechanisms of hormonal inductions in cultured hepatoma cells. J Biol Chem. 1970 Nov 10;245(21):5806–5812. [PubMed] [Google Scholar]
- Palmiter R. D. Rate of ovalbumin messenger ribonucleic acid synthesis in the oviduct of estrogen-primed chicks. J Biol Chem. 1973 Dec 10;248(23):8260–8270. [PubMed] [Google Scholar]
- Peterkofsky B., Tomkins G. M. Effect of inhibitors of nucleic acid synthesis on steroid-mediated induction of tyrosine aminotransferase in hepatoma cell cultures. J Mol Biol. 1967 Nov 28;30(1):49–61. doi: 10.1016/0022-2836(67)90242-2. [DOI] [PubMed] [Google Scholar]
- Rhoads R. E., McKnight G. S., Schimke R. T. Quantitative measurement of ovalbumin messenger ribonucleic acid activity. Localization in polysomes, induction by estrogen, and effect of actinomycin D. J Biol Chem. 1973 Mar 25;248(6):2031–2039. [PubMed] [Google Scholar]
- Rosenfeld G. C., Comstock J. P., Means A. R., O'Malley B. W. Estrogen-induced synthesis of ovalbumin messenger RNA and its translation in a cell-free system. Biochem Biophys Res Commun. 1972 Feb 25;46(4):1695–1703. doi: 10.1016/0006-291x(72)90805-4. [DOI] [PubMed] [Google Scholar]
- Schutz G., Beato M., Feigelson P. Messenger RNA for hepatic tryptophan oxygenase: its partial purification, its translation in a heterologous cell-free system, and its control by glucocorticoid hormones. Proc Natl Acad Sci U S A. 1973 Apr;70(4):1218–1221. doi: 10.1073/pnas.70.4.1218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott W. A., Shields R., Tomkins G. M. Mechanism of hormonal induction of tyrosine aminotransferase studied by measurement of the concentration of growing enzyme molecules. Proc Natl Acad Sci U S A. 1972 Oct;69(10):2937–2941. doi: 10.1073/pnas.69.10.2937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer R. H., Penman S. Messenger RNA in HeLa cells: kinetics of formation and decay. J Mol Biol. 1973 Aug 5;78(2):321–334. doi: 10.1016/0022-2836(73)90119-8. [DOI] [PubMed] [Google Scholar]
- Singer R. H., Penman S. Stability of HeLa cell mRNA in actinomycin. Nature. 1972 Nov 10;240(5376):100–102. doi: 10.1038/240100a0. [DOI] [PubMed] [Google Scholar]
- Thompson E. B., Tomkins G. M., Curran J. F. Induction of tyrosine alpha-ketoglutarate transaminase by steroid hormones in a newly established tissue culture cell line. Proc Natl Acad Sci U S A. 1966 Jul;56(1):296–303. doi: 10.1073/pnas.56.1.296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomkins G. M., Levinson B. B., Baxter J. D., Dethlefsen L. Further evidence for posttranscriptional control of inducible tyrosine aminotransferase synthesis in cultured hepatoma cells. Nat New Biol. 1972 Sep 6;239(88):9–14. doi: 10.1038/newbio239009a0. [DOI] [PubMed] [Google Scholar]
- Tomkins G. M., Thompson E. B., Hayashi S., Gelehrter T., Granner D., Peterkofsky B. Tyrosine transaminase induction in mammalian cells in tissue culture. Cold Spring Harb Symp Quant Biol. 1966;31:349–360. doi: 10.1101/sqb.1966.031.01.045. [DOI] [PubMed] [Google Scholar]


