Abstract
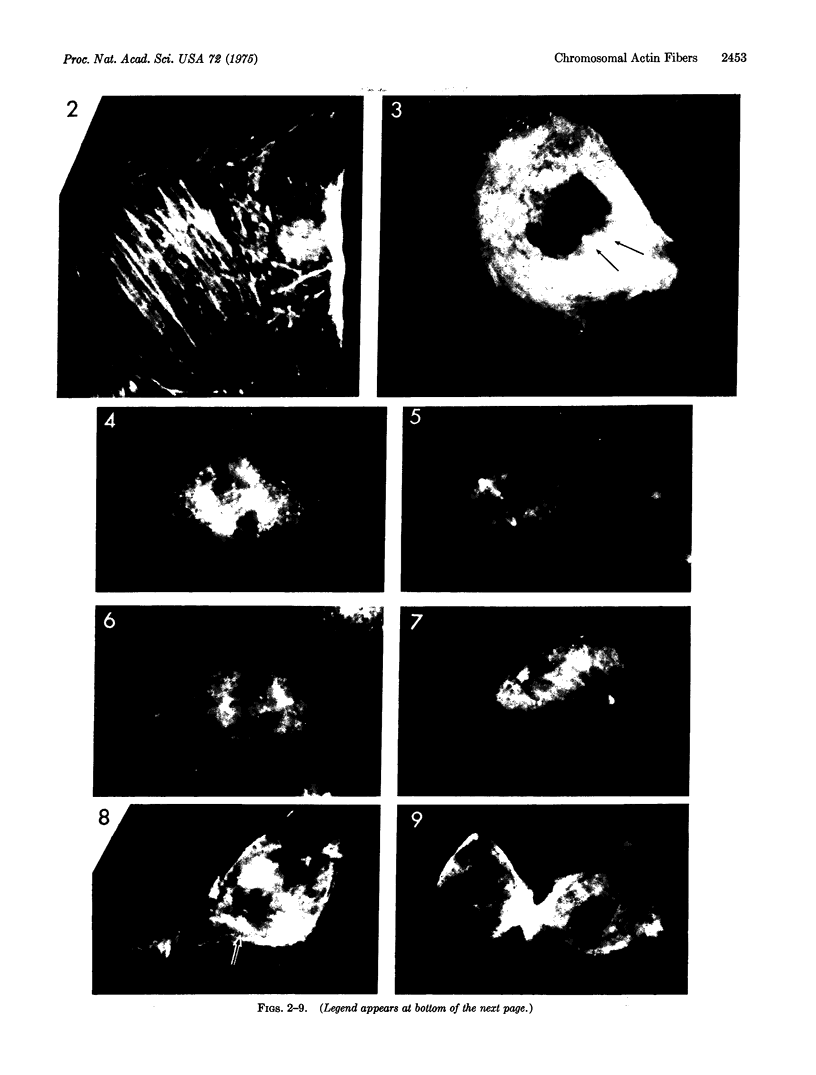
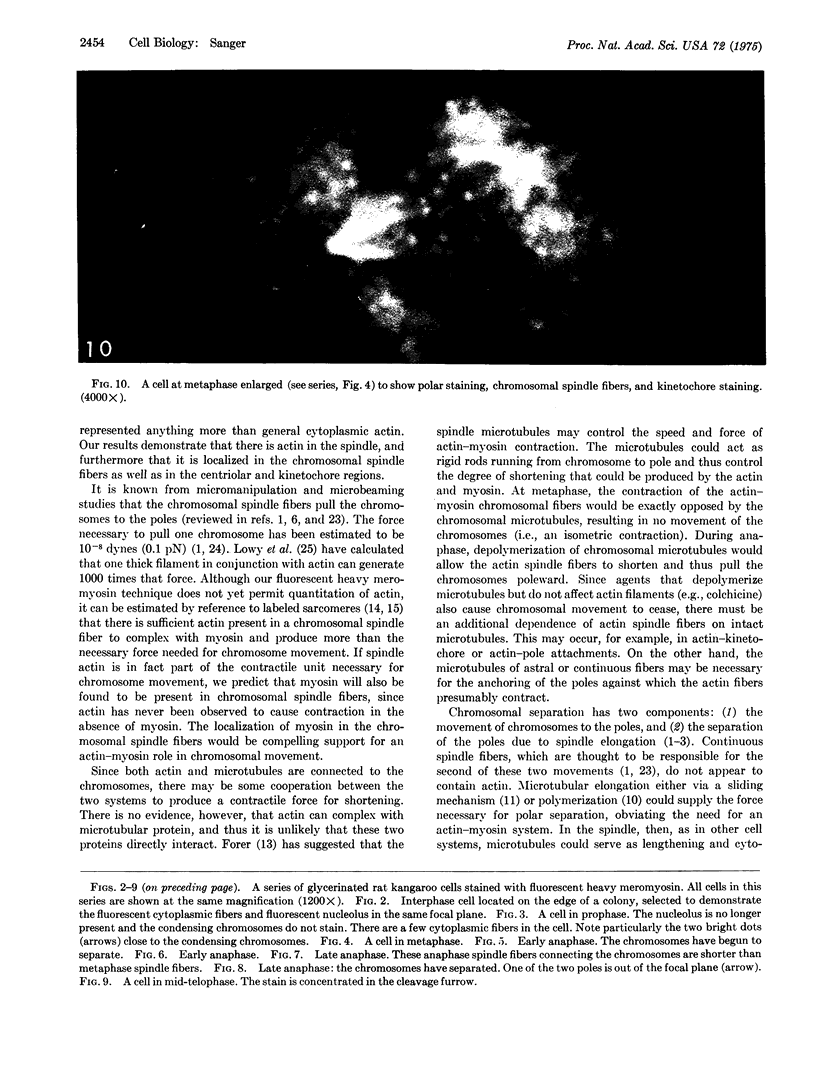
Actin has been shown to be present in the nucleoli, kinetochore and centriolar regions, and in the mitotic spindle of rat kangaroo cells which have been stained with fluorescently labeled heavy meromyosin. The actin in the spindle is confined to the fibers that connect the chromosomes with the centriolar region. Actin was not present in astral fibers, in the continuous spindle fibers that connect the poles, or in non-kinetochore regions of the chromosomes. The specific localization of actin in chromosomal spindle fibers suggests an actin-mvosin interaction as the force-producing mechanism for chromosomal movement.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BYERS B., PORTER K. R. ORIENTED MICROTUBULES IN ELONGATING CELLS OF THE DEVELOPING LENS RUDIMENT AFTER INDUCTION. Proc Natl Acad Sci U S A. 1964 Oct;52:1091–1099. doi: 10.1073/pnas.52.4.1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borisy G. G., Olmsted J. B., Klugman R. A. In vitro aggregation of cytoplasmic microtubule subunits. Proc Natl Acad Sci U S A. 1972 Oct;69(10):2890–2894. doi: 10.1073/pnas.69.10.2890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forer A., Behnke O. An actin-like component in spermatocytes of a crane fly (Nephrotoma suturalis Loew). I. The spindle. Chromosoma. 1972;39(2):145–173. doi: 10.1007/BF00319840. [DOI] [PubMed] [Google Scholar]
- Gawadi N. Actin in the mitotic spindle. Nature. 1971 Dec 17;234(5329):410–410. doi: 10.1038/234410a0. [DOI] [PubMed] [Google Scholar]
- Hinkley R., Telser A. Heavy meromyosin-binding filaments in the mitotic apparatus of mammaliam cells. Exp Cell Res. 1974 May;86(1):161–164. doi: 10.1016/0014-4827(74)90662-4. [DOI] [PubMed] [Google Scholar]
- Inoué S., Sato H. Cell motility by labile association of molecules. The nature of mitotic spindle fibers and their role in chromosome movement. J Gen Physiol. 1967 Jul;50(6 Suppl):259–292. [PMC free article] [PubMed] [Google Scholar]
- Ishikawa H., Bischoff R., Holtzer H. Mitosis and intermediate-sized filaments in developing skeletal muscle. J Cell Biol. 1968 Sep;38(3):538–555. doi: 10.1083/jcb.38.3.538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jockusch B. M., Becker M., Hindennach I., Jockusch H. Slime mould actin: homology to vertebrate actin and presence in the nucleus. Exp Cell Res. 1974 Dec;89(2):241–246. doi: 10.1016/0014-4827(74)90787-3. [DOI] [PubMed] [Google Scholar]
- Jockusch B. M., Brown D. F., Rusch H. P. Synthesis and some properties of an actin-like nuclear protein in the slime mold Physarum polycephalum. J Bacteriol. 1971 Nov;108(2):705–714. doi: 10.1128/jb.108.2.705-714.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWY J., MILLMAN B. M., HANSON J. STRUCTURE AND FUNCTION IN SMOOTH TONIC MUSCLES OF LAMELLIBRANCH MOLLUSCS. Proc R Soc Lond B Biol Sci. 1964 Oct 27;160:525–536. doi: 10.1098/rspb.1964.0068. [DOI] [PubMed] [Google Scholar]
- Lazarides E., Lindberg U. Actin is the naturally occurring inhibitor of deoxyribonuclease I. Proc Natl Acad Sci U S A. 1974 Dec;71(12):4742–4746. doi: 10.1073/pnas.71.12.4742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lestourgeon W. M., Forer A., Yang Y. Z., Bertram J. S., Pusch H. P. Contractile proteins. Major components of nuclear and chromosome non-histone proteins. Biochim Biophys Acta. 1975 Feb 27;379(2):529–552. [PubMed] [Google Scholar]
- McIntosh J. R., Landis S. C. The distribution of spindle microtubules during mitosis in cultured human cells. J Cell Biol. 1971 May 1;49(2):468–497. doi: 10.1083/jcb.49.2.468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molè-Bajer J. Fine structural studies of apolar mitosis. Chromosoma. 1969;26(4):427–448. doi: 10.1007/BF00326354. [DOI] [PubMed] [Google Scholar]
- Müller W. Elektronenmikroskopische Untersuchungen zum Formwechsel der Kinetochoren während der Spermatocytenteilungen von Pales ferruginea (Nematocera. Chromosoma. 1972;38(2):139–172. doi: 10.1007/BF00326191. [DOI] [PubMed] [Google Scholar]
- Nicklas R. B. Mitosis. Adv Cell Biol. 1971;2:225–297. doi: 10.1007/978-1-4615-9588-5_5. [DOI] [PubMed] [Google Scholar]
- Pollard T. D., Weihing R. R. Actin and myosin and cell movement. CRC Crit Rev Biochem. 1974 Jan;2(1):1–65. doi: 10.3109/10409237409105443. [DOI] [PubMed] [Google Scholar]
- Sanger J. M., Jackson W. T. Fine structure study of pollen development in Haemanthus katherinae Baker. II. Microtubules and elongation of the generative cells. J Cell Sci. 1971 Mar;8(2):303–315. doi: 10.1242/jcs.8.2.303. [DOI] [PubMed] [Google Scholar]
- Sanger J. W. Changing patterns of actin localization during cell division. Proc Natl Acad Sci U S A. 1975 May;72(5):1913–1916. doi: 10.1073/pnas.72.5.1913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ZIMMERMAN A. M., MARSLAND D. CELL DIVISION: EFFECTS OF PRESSURE ON THE MITOTIC MECHANISMS OF MARINE EGGS (ARBACIA PUNCTULATA). Exp Cell Res. 1964 Jul;35:293–302. doi: 10.1016/0014-4827(64)90096-5. [DOI] [PubMed] [Google Scholar]