Abstract
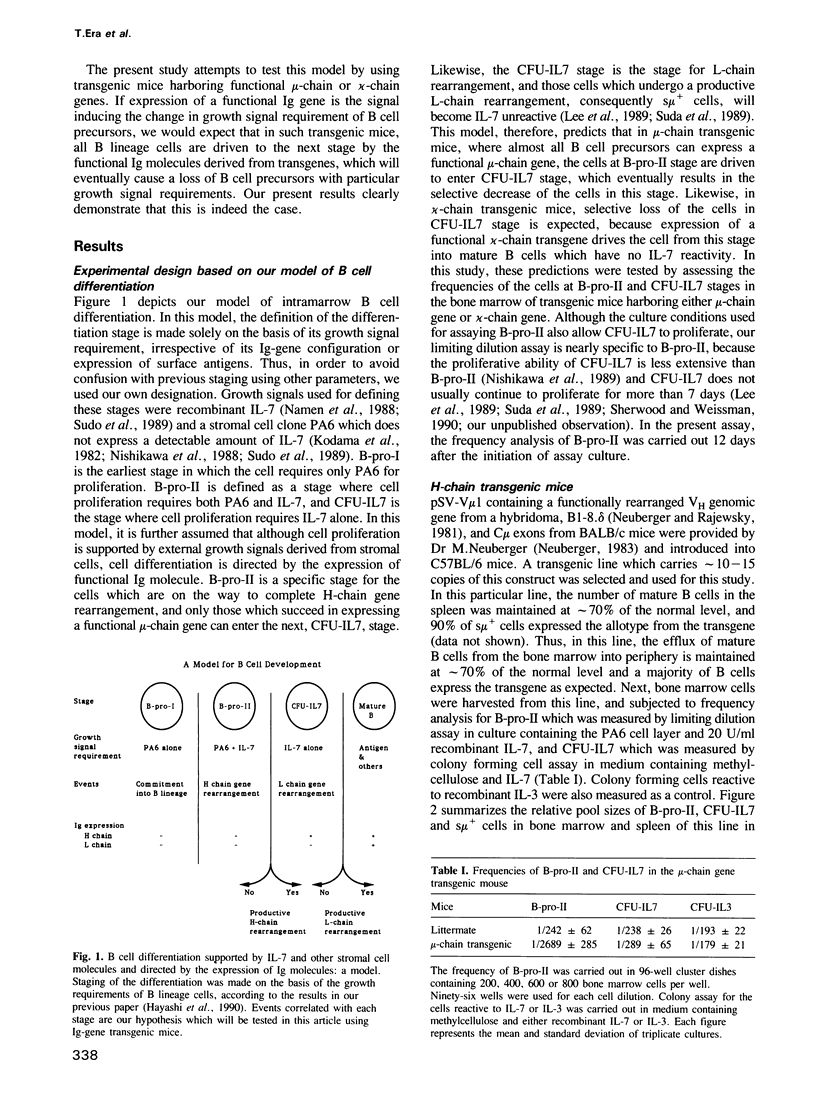
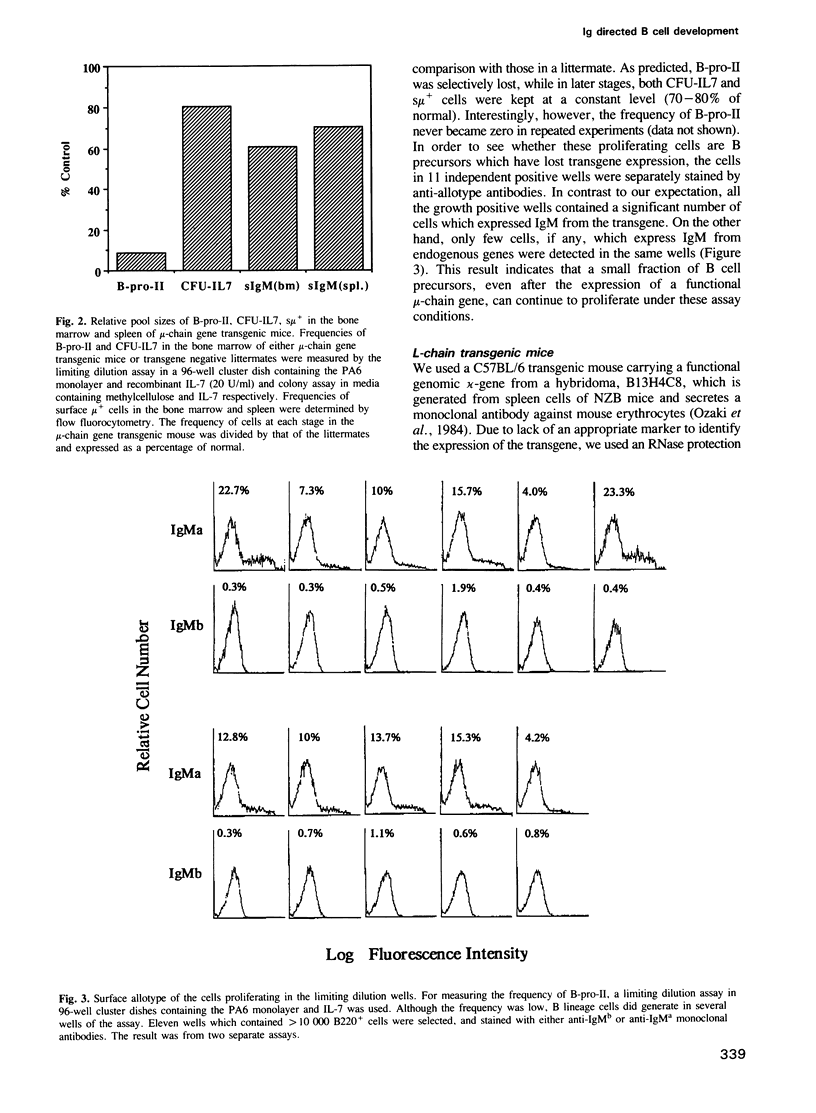
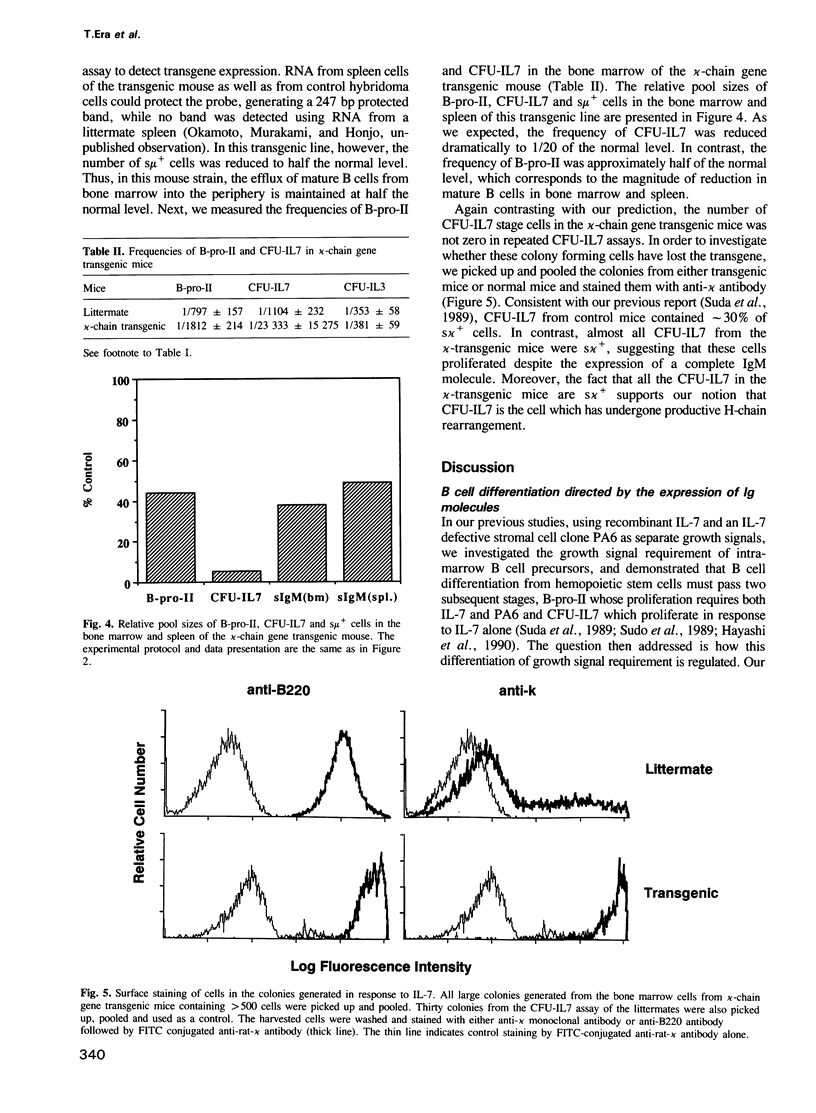
During B cell differentiation, at least three stages can be defined in terms of their growth signal requirement by using two different growth signals, which are recombinant interleukin 7 (IL-7) and a stromal cell clone PA6 which does not produce IL-7; first a PA6 dependent stage, second a PA6 + IL-7 dependent stage and third an IL-7 dependent stage. In order to test the possibility that this differentiation of growth signal requirement is controlled by the expression of functional immunoglobulin molecules, we have investigated the frequencies of PA6 + IL-7 dependent and IL-7 dependent cells which are present in the bone marrow of either mu-chain or kappa-chain gene transgenic mice. In a mu-chain gene transgenic mouse, the frequency of PA6 + IL-7 dependent cells is selectively reduced, while that of IL-7 dependent cells is selectively reduced in a kappa-chain gene transgenic mouse. This result suggests that expression of a functional mu-chain gene drives PA6 + IL-7 dependent cells to differentiate into the subsequent IL-7 dependent stage. Likewise, when mu-chain positive IL-7 dependent cells express a functional light-chain gene, their growth signal requirement changes into an IL-7 unreactive stage.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alt F. W., Baltimore D. Joining of immunoglobulin heavy chain gene segments: implications from a chromosome with evidence of three D-JH fusions. Proc Natl Acad Sci U S A. 1982 Jul;79(13):4118–4122. doi: 10.1073/pnas.79.13.4118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Araki K., Miyazaki J., Hino O., Tomita N., Chisaka O., Matsubara K., Yamamura K. Expression and replication of hepatitis B virus genome in transgenic mice. Proc Natl Acad Sci U S A. 1989 Jan;86(1):207–211. doi: 10.1073/pnas.86.1.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coffman R. L. Surface antigen expression and immunoglobulin gene rearrangement during mouse pre-B cell development. Immunol Rev. 1982;69:5–23. doi: 10.1111/j.1600-065x.1983.tb00446.x. [DOI] [PubMed] [Google Scholar]
- Coleclough C., Perry R. P., Karjalainen K., Weigert M. Aberrant rearrangements contribute significantly to the allelic exclusion of immunoglobulin gene expression. Nature. 1981 Apr 2;290(5805):372–378. doi: 10.1038/290372a0. [DOI] [PubMed] [Google Scholar]
- Early P., Huang H., Davis M., Calame K., Hood L. An immunoglobulin heavy chain variable region gene is generated from three segments of DNA: VH, D and JH. Cell. 1980 Apr;19(4):981–992. doi: 10.1016/0092-8674(80)90089-6. [DOI] [PubMed] [Google Scholar]
- Hayashi S., Kunisada T., Ogawa M., Sudo T., Kodama H., Suda T., Nishikawa S., Nishikawa S. Stepwise progression of B lineage differentiation supported by interleukin 7 and other stromal cell molecules. J Exp Med. 1990 May 1;171(5):1683–1695. doi: 10.1084/jem.171.5.1683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hendrickson E. A., Schatz D. G., Weaver D. T. The scid gene encodes a trans-acting factor that mediates the rejoining event of Ig gene rearrangement. Genes Dev. 1988 Jul;2(7):817–829. doi: 10.1101/gad.2.7.817. [DOI] [PubMed] [Google Scholar]
- Herzenberg L. A., Stall A. M., Braun J., Weaver D., Baltimore D., Herzenberg L. A., Grosschedl R. Depletion of the predominant B-cell population in immunoglobulin mu heavy-chain transgenic mice. Nature. 1987 Sep 3;329(6134):71–73. doi: 10.1038/329071a0. [DOI] [PubMed] [Google Scholar]
- Hombach J., Leclercq L., Radbruch A., Rajewsky K., Reth M. A novel 34-kd protein co-isolated with the IgM molecule in surface IgM-expressing cells. EMBO J. 1988 Nov;7(11):3451–3456. doi: 10.1002/j.1460-2075.1988.tb03219.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karasuyama H., Kudo A., Melchers F. The proteins encoded by the VpreB and lambda 5 pre-B cell-specific genes can associate with each other and with mu heavy chain. J Exp Med. 1990 Sep 1;172(3):969–972. doi: 10.1084/jem.172.3.969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kincade P. W. Experimental models for understanding B lymphocyte formation. Adv Immunol. 1987;41:181–267. doi: 10.1016/s0065-2776(08)60032-2. [DOI] [PubMed] [Google Scholar]
- Kodama H. A., Amagai Y., Koyama H., Kasai S. A new preadipose cell line derived from newborn mouse calvaria can promote the proliferation of pluripotent hemopoietic stem cells in vitro. J Cell Physiol. 1982 Jul;112(1):89–95. doi: 10.1002/jcp.1041120114. [DOI] [PubMed] [Google Scholar]
- Korsmeyer S. J., Hieter P. A., Ravetch J. V., Poplack D. G., Waldmann T. A., Leder P. Developmental hierarchy of immunoglobulin gene rearrangements in human leukemic pre-B-cells. Proc Natl Acad Sci U S A. 1981 Nov;78(11):7096–7100. doi: 10.1073/pnas.78.11.7096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kudo A., Melchers F. A second gene, VpreB in the lambda 5 locus of the mouse, which appears to be selectively expressed in pre-B lymphocytes. EMBO J. 1987 Aug;6(8):2267–2272. doi: 10.1002/j.1460-2075.1987.tb02500.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee G., Namen A. E., Gillis S., Ellingsworth L. R., Kincade P. W. Normal B cell precursors responsive to recombinant murine IL-7 and inhibition of IL-7 activity by transforming growth factor-beta. J Immunol. 1989 Jun 1;142(11):3875–3883. [PubMed] [Google Scholar]
- Lieber M. R., Hesse J. E., Lewis S., Bosma G. C., Rosenberg N., Mizuuchi K., Bosma M. J., Gellert M. The defect in murine severe combined immune deficiency: joining of signal sequences but not coding segments in V(D)J recombination. Cell. 1988 Oct 7;55(1):7–16. doi: 10.1016/0092-8674(88)90004-9. [DOI] [PubMed] [Google Scholar]
- Malynn B. A., Blackwell T. K., Fulop G. M., Rathbun G. A., Furley A. J., Ferrier P., Heinke L. B., Phillips R. A., Yancopoulos G. D., Alt F. W. The scid defect affects the final step of the immunoglobulin VDJ recombinase mechanism. Cell. 1988 Aug 12;54(4):453–460. doi: 10.1016/0092-8674(88)90066-9. [DOI] [PubMed] [Google Scholar]
- Müller W., Rüther U., Vieira P., Hombach J., Reth M., Rajewsky K. Membrane-bound IgM obstructs B cell development in transgenic mice. Eur J Immunol. 1989 May;19(5):923–928. doi: 10.1002/eji.1830190520. [DOI] [PubMed] [Google Scholar]
- Namen A. E., Lupton S., Hjerrild K., Wignall J., Mochizuki D. Y., Schmierer A., Mosley B., March C. J., Urdal D., Gillis S. Stimulation of B-cell progenitors by cloned murine interleukin-7. Nature. 1988 Jun 9;333(6173):571–573. doi: 10.1038/333571a0. [DOI] [PubMed] [Google Scholar]
- Neuberger M. S. Expression and regulation of immunoglobulin heavy chain gene transfected into lymphoid cells. EMBO J. 1983;2(8):1373–1378. doi: 10.1002/j.1460-2075.1983.tb01594.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neuberger M. S., Rajewsky K. Switch from hapten-specific immunoglobulin M to immunoglobulin D secretion in a hybrid mouse cell line. Proc Natl Acad Sci U S A. 1981 Feb;78(2):1138–1142. doi: 10.1073/pnas.78.2.1138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishikawa S., Hayashi S., Nishikawa S., Ogawa M., Kunisada T., Sudo T., Kodama H., Suda T. Defect of scid mouse revealed in in vitro culture systems. Curr Top Microbiol Immunol. 1989;152:39–46. doi: 10.1007/978-3-642-74974-2_6. [DOI] [PubMed] [Google Scholar]
- Nishikawa S., Ogawa M., Nishikawa S., Kunisada T., Kodama H. B lymphopoiesis on stromal cell clone: stromal cell clones acting on different stages of B cell differentiation. Eur J Immunol. 1988 Nov;18(11):1767–1771. doi: 10.1002/eji.1830181117. [DOI] [PubMed] [Google Scholar]
- Nishikawa S., Sasaki Y., Kina T., Amagai T., Katsura Y. A monoclonal antibody against Igh6-4 determinant. Immunogenetics. 1986;23(2):137–139. doi: 10.1007/BF00377976. [DOI] [PubMed] [Google Scholar]
- Nussenzweig M. C., Schmidt E. V., Shaw A. C., Sinn E., Campos-Torres J., Mathey-Prevot B., Pattengale P. K., Leder P. A human immunoglobulin gene reduces the incidence of lymphomas in c-Myc-bearing transgenic mice. Nature. 1988 Dec 1;336(6198):446–450. doi: 10.1038/336446a0. [DOI] [PubMed] [Google Scholar]
- Nussenzweig M. C., Shaw A. C., Sinn E., Campos-Torres J., Leder P. Allelic exclusion in transgenic mice carrying mutant human IgM genes. J Exp Med. 1988 Jun 1;167(6):1969–1974. doi: 10.1084/jem.167.6.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okazaki K., Nishikawa S., Sakano H. Aberrant immunoglobulin gene rearrangement in scid mouse bone marrow cells. J Immunol. 1988 Aug 15;141(4):1348–1352. [PubMed] [Google Scholar]
- Ozaki S., Nagasawa R., Sato H., Shirai T. Hybridoma autoantibodies to erythrocytes from NZB mice and the induction of hemolytic anemia. Immunol Lett. 1984;8(3):115–119. doi: 10.1016/0165-2478(84)90062-2. [DOI] [PubMed] [Google Scholar]
- Rath S., Durdik J., Gerstein R. M., Selsing E., Nisonoff A. Quantitative analysis of idiotypic mimicry and allelic exclusion in mice with a mu Ig transgene. J Immunol. 1989 Sep 15;143(6):2074–2080. [PubMed] [Google Scholar]
- Sakaguchi N., Kashiwamura S., Kimoto M., Thalmann P., Melchers F. B lymphocyte lineage-restricted expression of mb-1, a gene with CD3-like structural properties. EMBO J. 1988 Nov;7(11):3457–3464. doi: 10.1002/j.1460-2075.1988.tb03220.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakaguchi N., Melchers F. Lambda 5, a new light-chain-related locus selectively expressed in pre-B lymphocytes. Nature. 1986 Dec 11;324(6097):579–582. doi: 10.1038/324579a0. [DOI] [PubMed] [Google Scholar]
- Sakano H., Hüppi K., Heinrich G., Tonegawa S. Sequences at the somatic recombination sites of immunoglobulin light-chain genes. Nature. 1979 Jul 26;280(5720):288–294. doi: 10.1038/280288a0. [DOI] [PubMed] [Google Scholar]
- Sakano H., Maki R., Kurosawa Y., Roeder W., Tonegawa S. Two types of somatic recombination are necessary for the generation of complete immunoglobulin heavy-chain genes. Nature. 1980 Aug 14;286(5774):676–683. doi: 10.1038/286676a0. [DOI] [PubMed] [Google Scholar]
- Schuler W., Weiler I. J., Schuler A., Phillips R. A., Rosenberg N., Mak T. W., Kearney J. F., Perry R. P., Bosma M. J. Rearrangement of antigen receptor genes is defective in mice with severe combined immune deficiency. Cell. 1986 Sep 26;46(7):963–972. doi: 10.1016/0092-8674(86)90695-1. [DOI] [PubMed] [Google Scholar]
- Sherwood P. J., Weissman I. L. The growth factor IL-7 induces expression of a transformation-associated antigen in normal pre-B cells. Int Immunol. 1990;2(5):399–406. doi: 10.1093/intimm/2.5.399. [DOI] [PubMed] [Google Scholar]
- Stall A. M., Kroese F. G., Gadus F. T., Sieckmann D. G., Herzenberg L. A., Herzenberg L. A. Rearrangement and expression of endogenous immunoglobulin genes occur in many murine B cells expressing transgenic membrane IgM. Proc Natl Acad Sci U S A. 1988 May;85(10):3546–3550. doi: 10.1073/pnas.85.10.3546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suda T., Okada S., Suda J., Miura Y., Ito M., Sudo T., Hayashi S., Nishikawa S., Nakauchi H. A stimulatory effect of recombinant murine interleukin-7 (IL-7) on B-cell colony formation and an inhibitory effect of IL-1 alpha. Blood. 1989 Nov 1;74(6):1936–1941. [PubMed] [Google Scholar]
- Sudo T., Ito M., Ogawa Y., Iizuka M., Kodama H., Kunisada T., Hayashi S., Ogawa M., Sakai K., Nishikawa S. Interleukin 7 production and function in stromal cell-dependent B cell development. J Exp Med. 1989 Jul 1;170(1):333–338. doi: 10.1084/jem.170.1.333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsubata T., Reth M. The products of pre-B cell-specific genes (lambda 5 and VpreB) and the immunoglobulin mu chain form a complex that is transported onto the cell surface. J Exp Med. 1990 Sep 1;172(3):973–976. doi: 10.1084/jem.172.3.973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weaver D., Costantini F., Imanishi-Kari T., Baltimore D. A transgenic immunoglobulin mu gene prevents rearrangement of endogenous genes. Cell. 1985 Aug;42(1):117–127. doi: 10.1016/s0092-8674(85)80107-0. [DOI] [PubMed] [Google Scholar]
- Whitlock C. A., Ziegler S. F., Treiman L. J., Stafford J. I., Witte O. N. Differentiation of cloned populations of immature B cells after transformation with Abelson murine leukemia virus. Cell. 1983 Mar;32(3):903–911. doi: 10.1016/0092-8674(83)90075-2. [DOI] [PubMed] [Google Scholar]
- Yamamura K., Kikutani H., Takahashi N., Taga T., Akira S., Kawai K., Fukuchi K., Kumahara Y., Honjo T., Kishimoto T. Introduction of human gamma 1 immunoglobulin genes into fertilized mouse eggs. J Biochem. 1984 Aug;96(2):357–363. doi: 10.1093/oxfordjournals.jbchem.a134845. [DOI] [PubMed] [Google Scholar]
- Yelton D. E., Desaymard C., Scharff M. D. Use of monoclonal anti-mouse immunoglobulin to detect mouse antibodies. Hybridoma. 1981;1(1):5–11. doi: 10.1089/hyb.1.1981.1.5. [DOI] [PubMed] [Google Scholar]


