Abstract
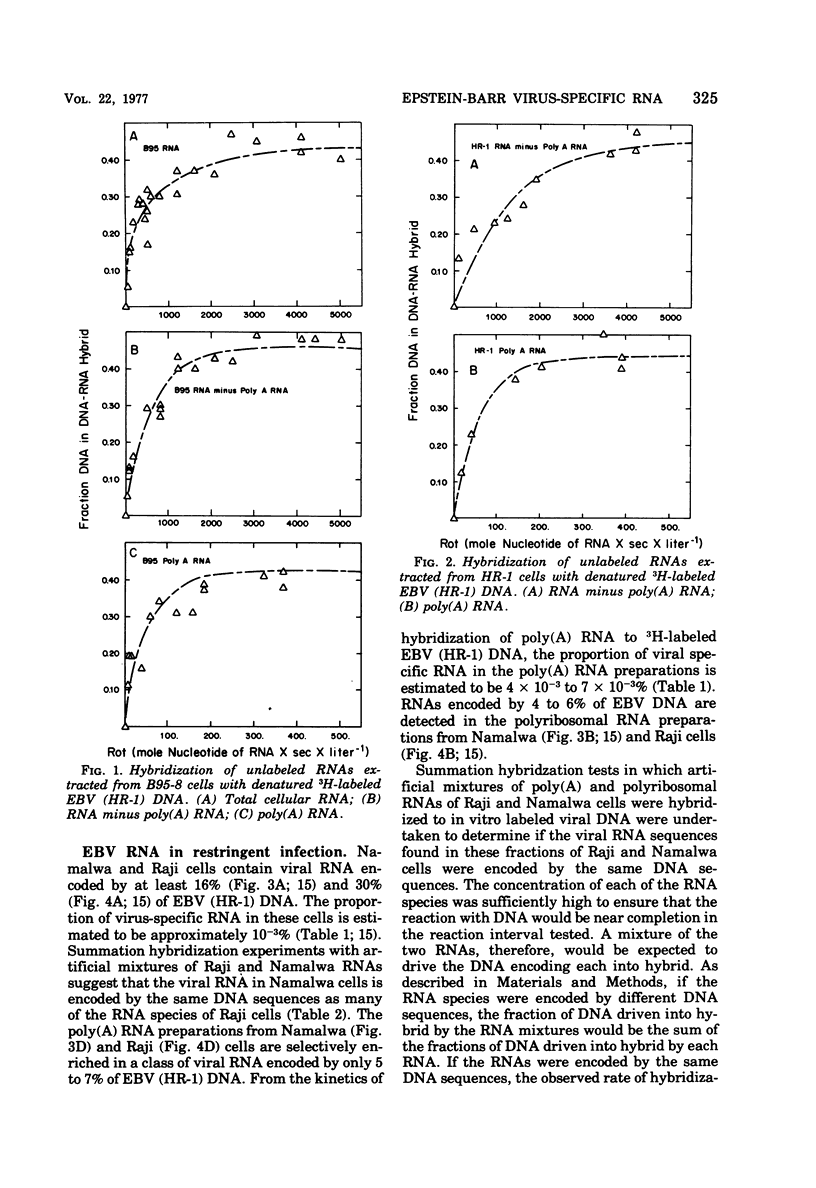
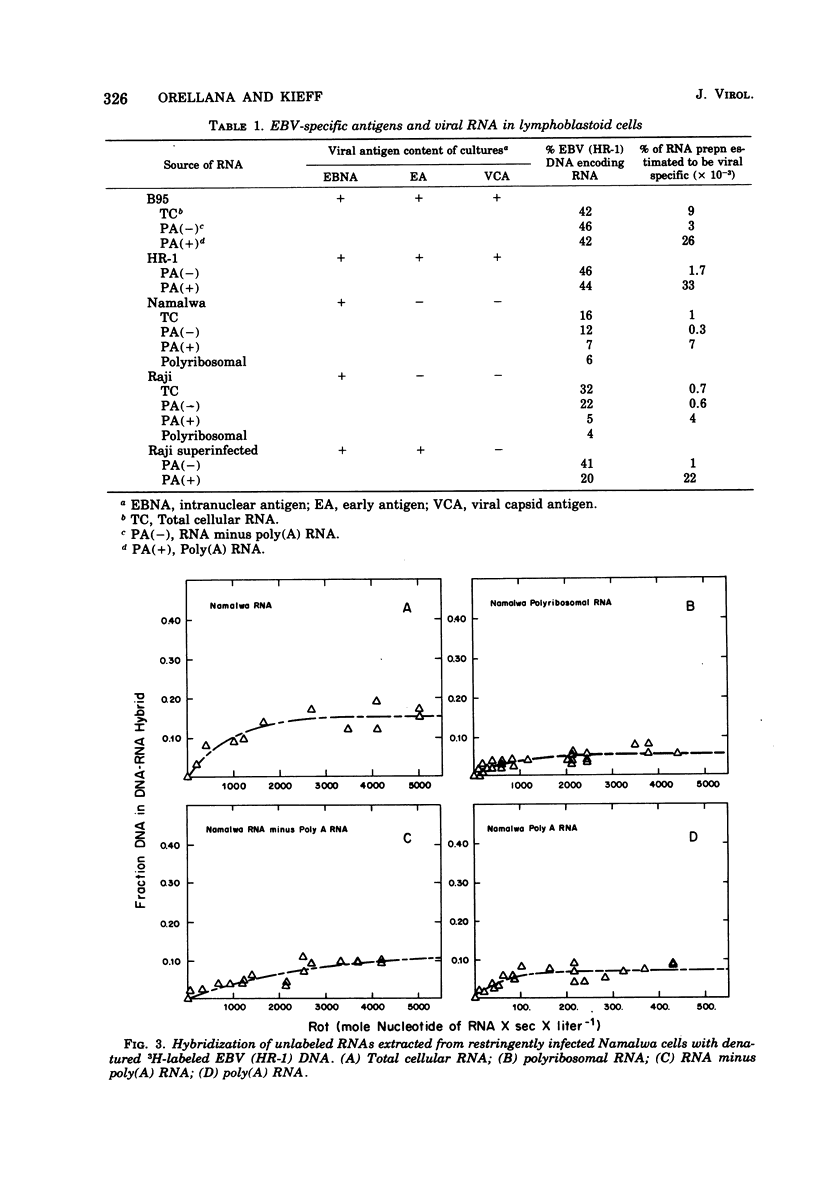
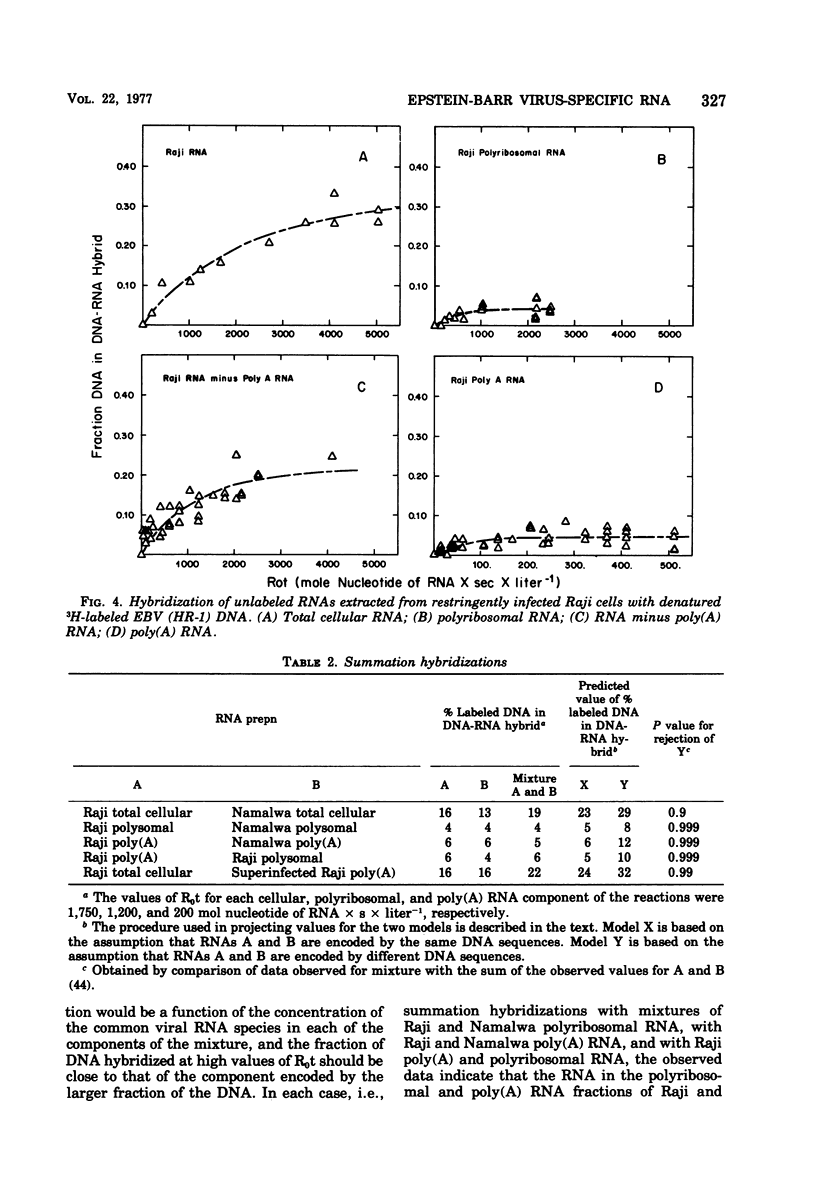
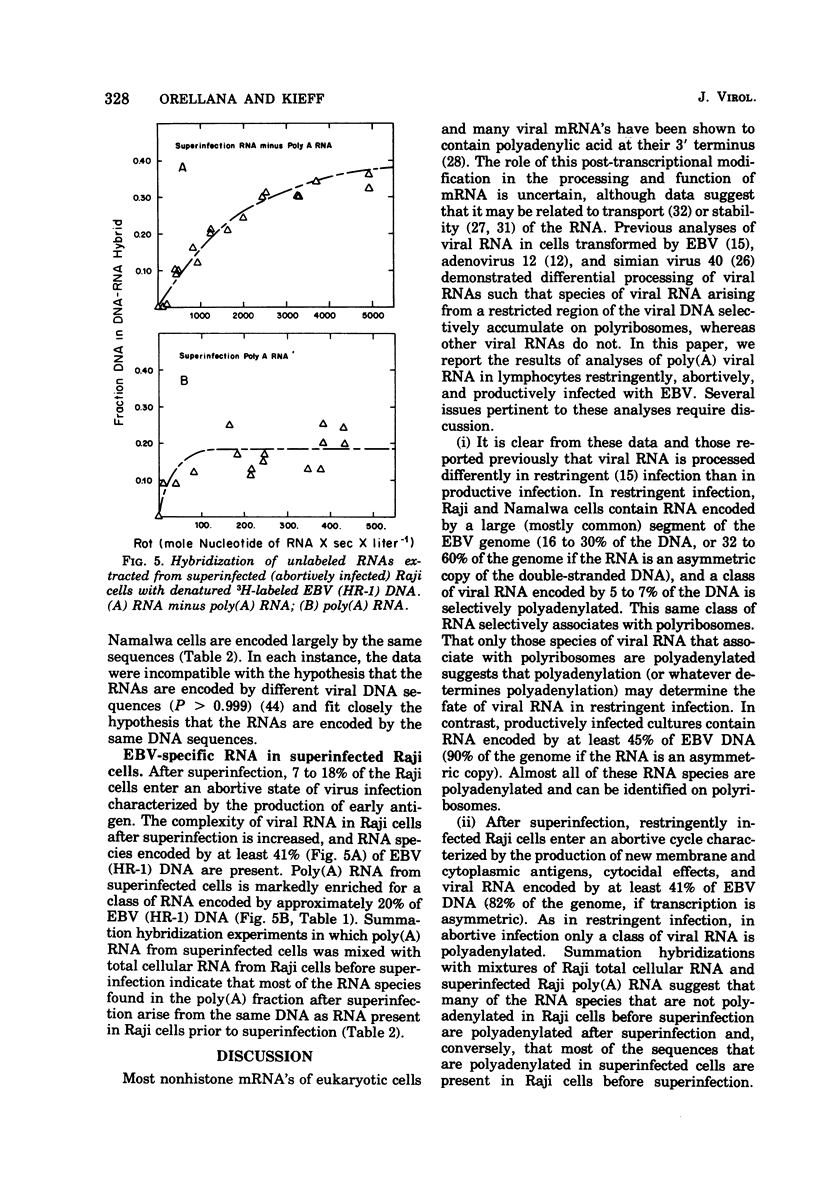
The complexity and abundance of Epstein-Barr (EBV)-specific RNA in cell cultures restringently, abortively, and productively infected with EBV has been analyed by hybridization of the infected cell RNA with purified viral DNA. The data indicate the following. (i) Cultures containing productively infected cells contain viral RNA encoded by at least 45% of EBV DNA, and almost all of the species of viral RNA are present in the polyadenylated and polyribosomal RNA fractions. (ii) Restringently infected Namalwa and Raji cultures, which contain only intranuclear antigen, EBNA, and enhanced capacity for growth in vitro, contain EBV RNA encoded by at least 16 and 30% of the EBV DNA, respectively. The polyadenylated and polyribosomal RNA fractions of Raji and Namalwa cells are enriched for a class of EBV RNA encoded by approximately 5% of EBV DNA. The same EBV DNA sequences encode the polyadenylated and polyribosomal RNA of both Raji and Namalwa cells. (iii) After superinfection of Raji cultures with EBV (HR-1), the abortively infected cells contain RNA encoded by at least 41% of EBV DNA. The polyadenylated RNA of superinfected Raji cells is enriched for a class of EBV RNA encoded by approximately 20% of EBV HR-1 DNA. Summation hybridization experiments suggest that the polyadenylated RNA in superinfected Raji cells is encoded by the same DNA sequences as encode RNA present in Raji cells before superinfection, most of which is not polyadenylated. That the same EBV RNA sequences are present in the polyadenylated and polyribosomal fractions of two independently derived, restringently infected cell lines suggests that these RNAs may specify functions related to maintenance of the transformed state. The complexity of this class of RNA is adequate to specify a sequence of a least 5,000 amino acids. That only some RNA species are polyadenylated in restringent and abortive infection suggests that polyadenylation or whatever determines polyadenylation may play a role in the restricted expression of the EVB genome.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aviv H., Leder P. Purification of biologically active globin messenger RNA by chromatography on oligothymidylic acid-cellulose. Proc Natl Acad Sci U S A. 1972 Jun;69(6):1408–1412. doi: 10.1073/pnas.69.6.1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubroff L. M., Nemer M. Molecular classes of heterogeneous nuclear RNA in sea urchin embryos. J Mol Biol. 1975 Jul 5;95(3):455–476. doi: 10.1016/0022-2836(75)90203-x. [DOI] [PubMed] [Google Scholar]
- EPSTEIN M. A., ACHONG B. G., BARR Y. M. VIRUS PARTICLES IN CULTURED LYMPHOBLASTS FROM BURKITT'S LYMPHOMA. Lancet. 1964 Mar 28;1(7335):702–703. doi: 10.1016/s0140-6736(64)91524-7. [DOI] [PubMed] [Google Scholar]
- Frenkel N., Roizman B. Ribonucleic acid synthesis in cells infected with herpes simplex virus: controls of transcription and of RNA abundance. Proc Natl Acad Sci U S A. 1972 Sep;69(9):2654–2658. doi: 10.1073/pnas.69.9.2654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerber P. Activation of Epstein-Barr virus by 5-bromodeoxyuridine in "virus-free" human cells (complement-fixing antigen-immunofluorescence-leukocytes). Proc Natl Acad Sci U S A. 1972 Jan;69(1):83–85. doi: 10.1073/pnas.69.1.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerber P., Monroe J. H. Studies on leukocytes growing in continuous culture derived from normal human donors. J Natl Cancer Inst. 1968 Apr;40(4):855–866. [PubMed] [Google Scholar]
- Gergely L., Klein G., Ernberg I. Appearance of Epstein-Barr virus-associated antigens in infected Raji cells. Virology. 1971 Jul;45(1):10–21. doi: 10.1016/0042-6822(71)90107-3. [DOI] [PubMed] [Google Scholar]
- Gergely L., Klein G., Ernberg I. Host cell macromolecular synthesis in cells containing EBV-induced early antigens, studied by combined immunofluorescence and radioautography. Virology. 1971 Jul;45(1):22–29. doi: 10.1016/0042-6822(71)90108-5. [DOI] [PubMed] [Google Scholar]
- Gergely L., Klein G., Ernberg I. The action of DNA antagonists on Epstein-Barr virus (EBV)-associated early antigen (EA) in Burkitt lymphoma lines. Int J Cancer. 1971 Mar 15;7(2):293–302. doi: 10.1002/ijc.2910070214. [DOI] [PubMed] [Google Scholar]
- Gerper P., Whang-Peng J., Monroe J. H. Transformation and chromosome changes induced by Epstein-Barr virus in normal human leukocyte cultures. Proc Natl Acad Sci U S A. 1969 Jul;63(3):740–747. doi: 10.1073/pnas.63.3.740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorski J., Morrison M. R., Merkel C. G., Lingrel J. B. Size heterogeneity of polyadenylate sequences in mouse globin messenger RNA. J Mol Biol. 1974 Jun 25;86(2):363–371. doi: 10.1016/0022-2836(74)90025-4. [DOI] [PubMed] [Google Scholar]
- Green M. R., Green M., Mackey J. K. Evidence for post-transcriptional selection of viral mRNA in cells transformed by human adenovirus 12. Nature. 1976 May 27;261(5558):340–342. doi: 10.1038/261340a0. [DOI] [PubMed] [Google Scholar]
- Hampar B., Derge J. G., Martos L. M., Walker J. L. Synthesis of Epstein-Barr virus after activation of the viral genome in a "virus-negative" human lymphoblastoid cell (Raji) made resistant to 5-bromodeoxyuridine (thymidine kinase-virus antigen-immunofluorescence-herpesvirus fingerprints). Proc Natl Acad Sci U S A. 1972 Jan;69(1):78–82. doi: 10.1073/pnas.69.1.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hampar B., Lenoir G., Nonoyama M., Derger J. G., Chang S. Cell cycle dependence for activation of Epstein-Barr virus by inhibitors of protein synthesis or medium deficient in arginine. Virology. 1976 Feb;69(2):660–668. doi: 10.1016/0042-6822(76)90494-3. [DOI] [PubMed] [Google Scholar]
- Hayward S. D., Kieff E. D. Epstein-Barr virus-specific RNA. I. Analysis of viral RNA in cellular extracts and in the polyribosomal fraction of permissive and nonpermissive lymphoblastoid cell lines. J Virol. 1976 May;18(2):518–525. doi: 10.1128/jvi.18.2.518-525.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henle G., Henle W. Immunofluorescence in cells derived from Burkitt's lymphoma. J Bacteriol. 1966 Mar;91(3):1248–1256. doi: 10.1128/jb.91.3.1248-1256.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henle W., Diehl V., Kohn G., Zur Hausen H., Henle G. Herpes-type virus and chromosome marker in normal leukocytes after growth with irradiated Burkitt cells. Science. 1967 Sep 1;157(3792):1064–1065. doi: 10.1126/science.157.3792.1064. [DOI] [PubMed] [Google Scholar]
- Henle W., Henle G., Zajac B. A., Pearson G., Waubke R., Scriba M. Differential reactivity of human serums with early antigens induced by Epstein-Barr virus. Science. 1970 Jul 10;169(3941):188–190. doi: 10.1126/science.169.3941.188. [DOI] [PubMed] [Google Scholar]
- Hinuma Y., Konn M., Yamaguchi J., Wudarski D. J., Blakeslee J. R., Jr, Grace J. T., Jr Immunofluorescence and herpes-type virus particles in the P3HR-1 Burkitt lymphoma cell line. J Virol. 1967 Oct;1(5):1045–1051. doi: 10.1128/jvi.1.5.1045-1051.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawai Y., Nonoyama M., Pagano J. S. Reassociation kinetics for Epstein-Barr virus DNA: nonhomology to mammalian DNA and homology of viral DNA in various diseases. J Virol. 1973 Nov;12(5):1006–1012. doi: 10.1128/jvi.12.5.1006-1012.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly R. B., Cozzarelli N. R., Deutscher M. P., Lehman I. R., Kornberg A. Enzymatic synthesis of deoxyribonucleic acid. XXXII. Replication of duplex deoxyribonucleic acid by polymerase at a single strand break. J Biol Chem. 1970 Jan 10;245(1):39–45. [PubMed] [Google Scholar]
- Kieff E., Levine J. Homology between Burkitt herpes viral DNA and DNA in continuous lymphoblastoid cells from patients with infectious mononucleosis. Proc Natl Acad Sci U S A. 1974 Feb;71(2):355–358. doi: 10.1073/pnas.71.2.355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenoir G., Berthelon M. C., Favre M. C., de-Thé G. Characterization of Epstein-Barr virus antigens. I. Biochemical analysis of the complement-fixing soluble antigen and relationship with Epstein-Barr virus-associated nuclear antigen. J Virol. 1976 Feb;17(2):672–674. doi: 10.1128/jvi.17.2.672-674.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leong J. A., Smith H. S., Goodman H. M. Viral RNA in the cytoplasm of SV40-transformed mouse cells: post-transcriptional processing. Virology. 1976 May;71(1):355–360. doi: 10.1016/0042-6822(76)90120-3. [DOI] [PubMed] [Google Scholar]
- Levy C. C., Schmukler M., Frank J. J., Karpetsky T. P., Jewett P. B., Hieter P. A., LeGendre S. M., Dorr R. G. Possible role for poly(A) as an inhibitor of endonuclease activity in eukaryotic cells. Nature. 1975 Jul 24;256(5515):340–342. doi: 10.1038/256340a0. [DOI] [PubMed] [Google Scholar]
- Lewin B. Units of transcription and translation: the relationship between heterogeneous nuclear RNA and messenger RNA. Cell. 1975 Jan;4(1):11–20. doi: 10.1016/0092-8674(75)90128-2. [DOI] [PubMed] [Google Scholar]
- Lindahl T., Klein G., Reedman B. M., Johansson B., Singh S. Relationship between Epstein-Barr virus (EBV) DNA and the EBV-determined nuclear antigen (EBNA) in Burkitt lymphoma biopsies and other lymphoproliferative malignancies. Int J Cancer. 1974 Jun 15;13(6):764–772. doi: 10.1002/ijc.2910130605. [DOI] [PubMed] [Google Scholar]
- Marbaix G., Huez G., Burny A., Cleuter Y., Hubert E., Leclercq M., Chantrenne H., Soreq H., Nudel U., Littauer U. Z. Absence of polyadenylate segment in globin messenger RNA accelerates its degradation in Xenopus oocytes. Proc Natl Acad Sci U S A. 1975 Aug;72(8):3065–3067. doi: 10.1073/pnas.72.8.3065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McConaughy B. L., Laird C. D., McCarthy B. J. Nucleic acid reassociation in formamide. Biochemistry. 1969 Aug;8(8):3289–3295. doi: 10.1021/bi00836a024. [DOI] [PubMed] [Google Scholar]
- Milcarek C., Penman S. Membrane-bound polyribosomes in HeLa cells: association of polyadenylic acid with membranes. J Mol Biol. 1974 Oct 25;89(2):327–338. doi: 10.1016/0022-2836(74)90522-1. [DOI] [PubMed] [Google Scholar]
- Miller G., Shope T., Lisco H., Stitt D., Lipman M. Epstein-Barr virus: transformation, cytopathic changes, and viral antigens in squirrel monkey and marmoset leukocytes. Proc Natl Acad Sci U S A. 1972 Feb;69(2):383–387. doi: 10.1073/pnas.69.2.383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nonoyama M., Pagano J. S. Homology between Epstein-Barr virus DNA and viral DNA from Burkitt's lymphoma and nasopharyngeal carcinoma determined by DNA-DNA reassociation kinetics. Nature. 1973 Mar 2;242(5392):44–47. doi: 10.1038/242044a0. [DOI] [PubMed] [Google Scholar]
- Pagano J. S., Huang C. H., Levine P. Absence of Epstein-Barr viral DNA in Amercian Burkitt's lymphoma. N Engl J Med. 1973 Dec 27;289(26):1395–1399. doi: 10.1056/NEJM197312272892604. [DOI] [PubMed] [Google Scholar]
- Perry R. P., La Torre J., Kelley D. E., Greenberg J. R. On the lability of poly(A) sequences during extraction of messenger RNA from polyribosomes. Biochim Biophys Acta. 1972 Mar 14;262(2):220–226. doi: 10.1016/0005-2787(72)90236-5. [DOI] [PubMed] [Google Scholar]
- Pope J. H. Establishment of cell lines from peripheral leucocytes in infectious mononucleosis. Nature. 1967 Nov 25;216(5117):810–811. doi: 10.1038/216810a0. [DOI] [PubMed] [Google Scholar]
- Pope J. H., Horne M. K., Scott W. Transformation of foetal human keukocytes in vitro by filtrates of a human leukaemic cell line containing herpes-like virus. Int J Cancer. 1968 Nov 15;3(6):857–866. doi: 10.1002/ijc.2910030619. [DOI] [PubMed] [Google Scholar]
- Pritchett R. F., Hayward S. D., Kieff E. D. DNA of Epstein-Barr virus. I. Comparative studies of the DNA of Epstein-Barr virus from HR-1 and B95-8 cells: size, structure, and relatedness. J Virol. 1975 Mar;15(3):556–559. doi: 10.1128/jvi.15.3.556-559.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pritchett R. P., Hayward S. D., Kieff E. D. Analysis of the DNA OF Epstein-Barr viruses and transcriptional products in transformed cells. IARC Sci Publ. 1975;(11 Pt 1):177–189. [PubMed] [Google Scholar]
- Pritchett R., Pendersen M., Kieff E. Complexity of EBV homologous DNA in continous lymphoblastoid cell lines. Virology. 1976 Oct 1;74(1):227–231. [PubMed] [Google Scholar]
- Reedman B. M., Klein G., Pope J. H., Walters M. K., Hilgers J., Singh S., Johansson B. Epstein-Barr virus-associated complement-fixing and nuclear antigens in Burkitt lymphoma biopsies. Int J Cancer. 1974 Jun 15;13(6):755–763. doi: 10.1002/ijc.2910130604. [DOI] [PubMed] [Google Scholar]
- Singer R. H., Penman S. Messenger RNA in HeLa cells: kinetics of formation and decay. J Mol Biol. 1973 Aug 5;78(2):321–334. doi: 10.1016/0022-2836(73)90119-8. [DOI] [PubMed] [Google Scholar]
- Steinitz M., Klein G. Epstein-Barr virus (EBV)-induced change in the saturation sensitivity and serum dependence of established, EBV-negative lymphoma lines in vitro. Virology. 1976 Apr;70(2):570–573. doi: 10.1016/0042-6822(76)90302-0. [DOI] [PubMed] [Google Scholar]
- Sutton W. D. A crude nuclease preparation suitable for use in DNA reassociation experiments. Biochim Biophys Acta. 1971 Jul 29;240(4):522–531. doi: 10.1016/0005-2787(71)90709-x. [DOI] [PubMed] [Google Scholar]
- Thorley-Lawson D., Strominger J. L. Transformation of human lymphocytes by Epstein-Barr virus is inhibited by phosphonoacetic acid. Nature. 1976 Sep 23;263(5575):332–334. doi: 10.1038/263332a0. [DOI] [PubMed] [Google Scholar]
- Wiegand R. C., Godson G. N., Radding C. M. Specificity of the S1 nuclease from Aspergillus oryzae. J Biol Chem. 1975 Nov 25;250(22):8848–8855. [PubMed] [Google Scholar]
- Willard J. M., Davis J. J., Wood H. G. Phosphoenolpyruvate carboxytransphosphorylase. IV. Requirement for metal cations. Biochemistry. 1969 Aug;8(8):3137–3144. doi: 10.1021/bi00836a002. [DOI] [PubMed] [Google Scholar]
- Yajima Y., Nonoyama M. Mechanisms of infection with Epstein-Barr virus. I. Viral DNA replication and formation of noninfectious virus particles in superinfected Raji cells. J Virol. 1976 Jul;19(1):187–194. doi: 10.1128/jvi.19.1.187-194.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmerman S. B., Sandeen D. The ribonuclease activity of crystallized pancreatic deoxyribonuclease. Anal Biochem. 1966 Feb;14(2):269–277. doi: 10.1016/0003-2697(66)90137-0. [DOI] [PubMed] [Google Scholar]


