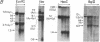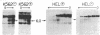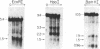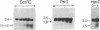Abstract
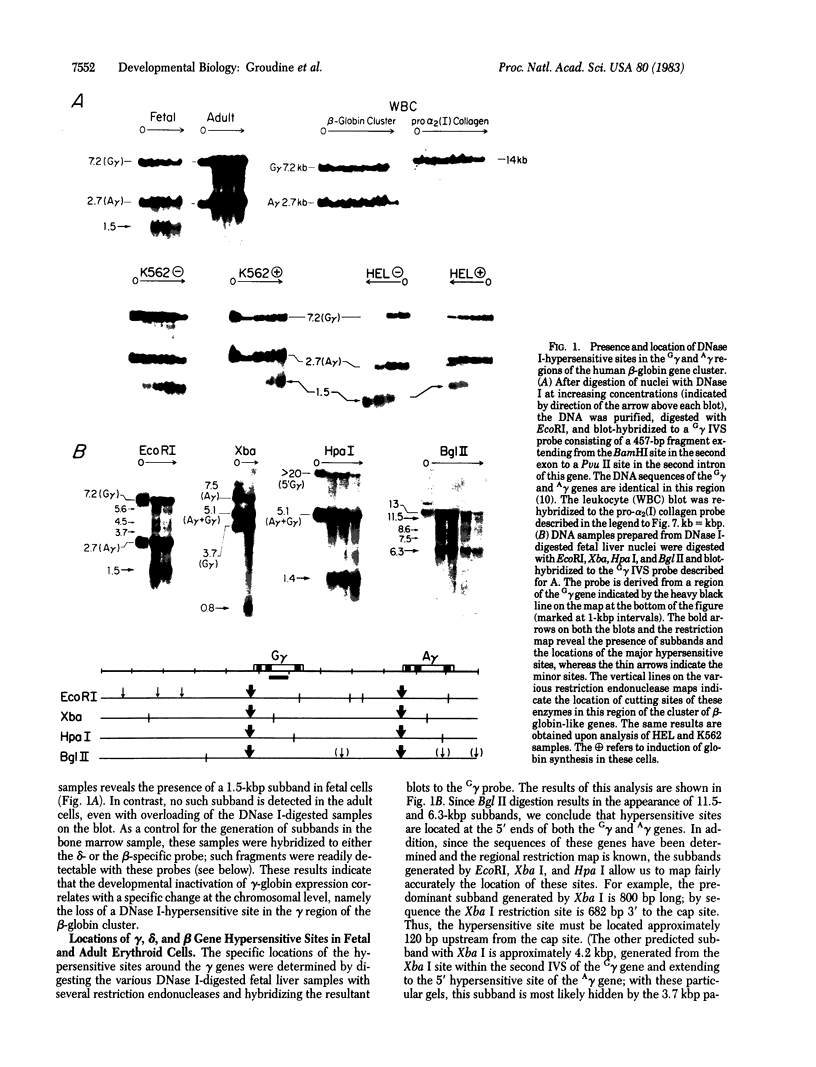
We have investigated the chromatin structure of the chromosomal DNA regions containing the human G gamma-, A gamma-, delta-, and beta-globin structural genes in both fetal and adult erythropoietic tissues and in two human erythroleukemia cells lines before and after induction. Our results indicate that DNase I introduces specific cuts into the beta-globin gene cluster in erythroid cells but not in leukocytes. The predominant sites are located at the 5' sides of the G gamma-, A gamma-, delta-, and beta-globin genes, within 200 base pairs of the respective cap sites. Examination of fetal liver cells has revealed the presence of hypersensitive sites at the 5' side of all four genes, whereas analysis of adult bone marrow has revealed the characteristic sites near the delta- and beta-globin genes but no hypersensitive sites at the 5' termini of the G gamma- or A gamma-globin genes. The presence of delta and beta hypersensitive sites in fetal cells suggests that the increment in expression of the delta and beta genes during development most likely involves the modulation of another pathway to gene expression. Using isolated nuclei from HEL and K562 cells, we have found that the G gamma, A gamma, delta, and beta genes are preferentially sensitive [relative to the pro-alpha2(I) collagen gene] to mild digestion with DNase I, whereas these genes are as resistant as collagen genes in cells that do not express globin. These findings are discussed within the context of chromatin structural correlates of hemoglobin switching.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Charnay P., Maniatis T. Transcriptional regulation of globin gene expression in the human erythroid cell line K562. Science. 1983 Jun 17;220(4603):1281–1283. doi: 10.1126/science.6574602. [DOI] [PubMed] [Google Scholar]
- Collins S. J., Gallo R. C., Gallagher R. E. Continuous growth and differentiation of human myeloid leukaemic cells in suspension culture. Nature. 1977 Nov 24;270(5635):347–349. doi: 10.1038/270347a0. [DOI] [PubMed] [Google Scholar]
- Dean A., Ley T. J., Humphries R. K., Fordis M., Schechter A. N. Inducible transcription of five globin genes in K562 human leukemia cells. Proc Natl Acad Sci U S A. 1983 Sep;80(18):5515–5519. doi: 10.1073/pnas.80.18.5515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elgin S. C. DNAase I-hypersensitive sites of chromatin. Cell. 1981 Dec;27(3 Pt 2):413–415. doi: 10.1016/0092-8674(81)90381-0. [DOI] [PubMed] [Google Scholar]
- Groudine M., Peretz M., Weintraub H. Transcriptional regulation of hemoglobin switching in chicken embryos. Mol Cell Biol. 1981 Mar;1(3):281–288. doi: 10.1128/mcb.1.3.281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groudine M., Weintraub H. Propagation of globin DNAase I-hypersensitive sites in absence of factors required for induction: a possible mechanism for determination. Cell. 1982 Aug;30(1):131–139. doi: 10.1016/0092-8674(82)90019-8. [DOI] [PubMed] [Google Scholar]
- Hofer E., Darnell J. E., Jr The primary transcription unit of the mouse beta-major globin gene. Cell. 1981 Feb;23(2):585–593. doi: 10.1016/0092-8674(81)90154-9. [DOI] [PubMed] [Google Scholar]
- Kafatos F. C., Jones C. W., Efstratiadis A. Determination of nucleic acid sequence homologies and relative concentrations by a dot hybridization procedure. Nucleic Acids Res. 1979 Nov 24;7(6):1541–1552. doi: 10.1093/nar/7.6.1541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawn R. M., Efstratiadis A., O'Connell C., Maniatis T. The nucleotide sequence of the human beta-globin gene. Cell. 1980 Oct;21(3):647–651. doi: 10.1016/0092-8674(80)90428-6. [DOI] [PubMed] [Google Scholar]
- Lozzio C. B., Lozzio B. B. Human chronic myelogenous leukemia cell-line with positive Philadelphia chromosome. Blood. 1975 Mar;45(3):321–334. [PubMed] [Google Scholar]
- Maniatis T., Fritsch E. F., Lauer J., Lawn R. M. The molecular genetics of human hemoglobins. Annu Rev Genet. 1980;14:145–178. doi: 10.1146/annurev.ge.14.120180.001045. [DOI] [PubMed] [Google Scholar]
- Martin P., Papayannopoulou T. HEL cells: a new human erythroleukemia cell line with spontaneous and induced globin expression. Science. 1982 Jun 11;216(4551):1233–1235. doi: 10.1126/science.6177045. [DOI] [PubMed] [Google Scholar]
- Rutherford T. R., Clegg J. B., Weatherall D. J. K562 human leukaemic cells synthesise embryonic haemoglobin in response to haemin. Nature. 1979 Jul 12;280(5718):164–165. doi: 10.1038/280164a0. [DOI] [PubMed] [Google Scholar]
- Shen C. K., Maniatis T. The organization of repetitive sequences in a cluster of rabbit beta-like globin genes. Cell. 1980 Feb;19(2):379–391. doi: 10.1016/0092-8674(80)90512-7. [DOI] [PubMed] [Google Scholar]
- Shen S. H., Slightom J. L., Smithies O. A history of the human fetal globin gene duplication. Cell. 1981 Oct;26(2 Pt 2):191–203. doi: 10.1016/0092-8674(81)90302-0. [DOI] [PubMed] [Google Scholar]
- Spritz R. A., DeRiel J. K., Forget B. G., Weissman S. M. Complete nucleotide sequence of the human delta-globin gene. Cell. 1980 Oct;21(3):639–646. doi: 10.1016/0092-8674(80)90427-4. [DOI] [PubMed] [Google Scholar]
- Stahl S. J., Chamberlin M. J. Transcription of T7 DNA containing modified nucleotides by bacteriophage T7 specific RNA polymerase. J Biol Chem. 1978 Jul 25;253(14):4951–4959. [PubMed] [Google Scholar]
- Stalder J., Larsen A., Engel J. D., Dolan M., Groudine M., Weintraub H. Tissue-specific DNA cleavages in the globin chromatin domain introduced by DNAase I. Cell. 1980 Jun;20(2):451–460. doi: 10.1016/0092-8674(80)90631-5. [DOI] [PubMed] [Google Scholar]
- Weintraub H., Larsen A., Groudine M. Alpha-Globin-gene switching during the development of chicken embryos: expression and chromosome structure. Cell. 1981 May;24(2):333–344. doi: 10.1016/0092-8674(81)90323-8. [DOI] [PubMed] [Google Scholar]
- Wu C. The 5' ends of Drosophila heat shock genes in chromatin are hypersensitive to DNase I. Nature. 1980 Aug 28;286(5776):854–860. doi: 10.1038/286854a0. [DOI] [PubMed] [Google Scholar]