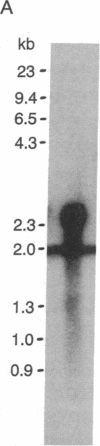
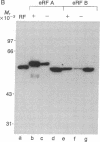
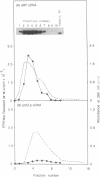
Abstract
The termination of protein synthesis is encoded by in-frame nonsense (stop) codons. Most organisms use three nonsense codons: UGA, UAG, and UAA. In contrast to sense codons, which are decoded by specific tRNAs, nonsense codons are decoded by proteins called release factors (RFs). Here we report the cloning of a mammalian RF cDNA by the use of monoclonal antibodies specific for rabbit RF. Functional studies showed that, when expressed in Escherichia coli, the protein encoded by this cDNA has in vitro biochemical characteristics similar to those of previously characterized mammalian RFs. DNA sequencing of this eukaryotic RF cDNA revealed a remarkable sequence similarity to bacterial and mitochondrial tryptophanyl-tRNA synthetases, with the greatest similarity confined to the synthetase active site, and no obvious similarity to bacterial RFs.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barstow D. A., Sharman A. F., Atkinson T., Minton N. P. Cloning and complete nucleotide sequence of the Bacillus stearothermophilus tryptophanyl tRNA synthetase gene. Gene. 1986;46(1):37–45. doi: 10.1016/0378-1119(86)90164-2. [DOI] [PubMed] [Google Scholar]
- Beaudet A. L., Caskey C. T. Mammalian peptide chain termination. II. Codon specificity and GTPase activity of release factor. Proc Natl Acad Sci U S A. 1971 Mar;68(3):619–624. doi: 10.1073/pnas.68.3.619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breton R., Sanfaçon H., Papayannopoulos I., Biemann K., Lapointe J. Glutamyl-tRNA synthetase of Escherichia coli. Isolation and primary structure of the gltX gene and homology with other aminoacyl-tRNA synthetases. J Biol Chem. 1986 Aug 15;261(23):10610–10617. [PubMed] [Google Scholar]
- Capecchi M. R., Klein H. A. Release factors mediating termination of complete proteins. Nature. 1970 Jun 13;226(5250):1029–1033. doi: 10.1038/2261029a0. [DOI] [PubMed] [Google Scholar]
- Caskey C. T., Beaudet A. L., Tate W. P. Mammalian release factor; in vitro assay and purification. Methods Enzymol. 1974;30:293–303. doi: 10.1016/0076-6879(74)30032-8. [DOI] [PubMed] [Google Scholar]
- Chen C., Okayama H. High-efficiency transformation of mammalian cells by plasmid DNA. Mol Cell Biol. 1987 Aug;7(8):2745–2752. doi: 10.1128/mcb.7.8.2745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craigen W. J., Cook R. G., Tate W. P., Caskey C. T. Bacterial peptide chain release factors: conserved primary structure and possible frameshift regulation of release factor 2. Proc Natl Acad Sci U S A. 1985 Jun;82(11):3616–3620. doi: 10.1073/pnas.82.11.3616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crick F. H. The origin of the genetic code. J Mol Biol. 1968 Dec;38(3):367–379. doi: 10.1016/0022-2836(68)90392-6. [DOI] [PubMed] [Google Scholar]
- Goldstein J. L., Beaudet A. L., Caskey C. T. Peptide chain termination with mammalian release factor. Proc Natl Acad Sci U S A. 1970 Sep;67(1):99–106. doi: 10.1073/pnas.67.1.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gubler U., Hoffman B. J. A simple and very efficient method for generating cDNA libraries. Gene. 1983 Nov;25(2-3):263–269. doi: 10.1016/0378-1119(83)90230-5. [DOI] [PubMed] [Google Scholar]
- Hall C. V., vanCleemput M., Muench K. H., Yanofsky C. The nucleotide sequence of the structural gene for Escherichia coli tryptophanyl-tRNA synthetase. J Biol Chem. 1982 Jun 10;257(11):6132–6136. [PubMed] [Google Scholar]
- Hanyu N., Kuchino Y., Nishimura S., Beier H. Dramatic events in ciliate evolution: alteration of UAA and UAG termination codons to glutamine codons due to anticodon mutations in two Tetrahymena tRNAs. EMBO J. 1986 Jun;5(6):1307–1311. doi: 10.1002/j.1460-2075.1986.tb04360.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henikoff S. Unidirectional digestion with exonuclease III creates targeted breakpoints for DNA sequencing. Gene. 1984 Jun;28(3):351–359. doi: 10.1016/0378-1119(84)90153-7. [DOI] [PubMed] [Google Scholar]
- Hountondji C., Blanquet S., Lederer F. Methionyl-tRNA synthetase from Escherichia coli: primary structure at the binding site for the 3'-end of tRNAfMet. Biochemistry. 1985 Feb 26;24(5):1175–1180. doi: 10.1021/bi00326a018. [DOI] [PubMed] [Google Scholar]
- Hountondji C., Dessen P., Blanquet S. Sequence similarities among the family of aminoacyl-tRNA synthetases. Biochimie. 1986 Sep;68(9):1071–1078. doi: 10.1016/s0300-9084(86)80181-x. [DOI] [PubMed] [Google Scholar]
- Hountondji C., Lederer F., Dessen P., Blanquet S. Escherichia coli tyrosyl- and methionyl-tRNA synthetases display sequence similarity at the binding site for the 3'-end of tRNA. Biochemistry. 1986 Jan 14;25(1):16–21. doi: 10.1021/bi00349a003. [DOI] [PubMed] [Google Scholar]
- Keng T., Webster T. A., Sauer R. T., Schimmel P. Gene for Escherichia coli glycyl-tRNA synthetase has tandem subunit coding regions in the same reading frame. J Biol Chem. 1982 Nov 10;257(21):12503–12508. [PubMed] [Google Scholar]
- Konecki D. S., Aune K. C., Tate W., Caskey C. T. Characterization of reticulocyte release factor. J Biol Chem. 1977 Jul 10;252(13):4514–4520. [PubMed] [Google Scholar]
- Labouesse M., Herbert C. J., Dujardin G., Slonimski P. P. Three suppressor mutations which cure a mitochondrial RNA maturase deficiency occur at the same codon in the open reading frame of the nuclear NAM2 gene. EMBO J. 1987 Mar;6(3):713–721. doi: 10.1002/j.1460-2075.1987.tb04812.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee C. C., Timms K. M., Trotman C. N., Tate W. P. Isolation of a rat mitochondrial release factor. Accommodation of the changed genetic code for termination. J Biol Chem. 1987 Mar 15;262(8):3548–3552. [PubMed] [Google Scholar]
- Lee C. C., Wu X. W., Gibbs R. A., Cook R. G., Muzny D. M., Caskey C. T. Generation of cDNA probes directed by amino acid sequence: cloning of urate oxidase. Science. 1988 Mar 11;239(4845):1288–1291. doi: 10.1126/science.3344434. [DOI] [PubMed] [Google Scholar]
- Martinez H. M. A flexible multiple sequence alignment program. Nucleic Acids Res. 1988 Mar 11;16(5):1683–1691. doi: 10.1093/nar/16.5.1683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mierendorf R. C., Percy C., Young R. A. Gene isolation by screening lambda gt11 libraries with antibodies. Methods Enzymol. 1987;152:458–469. doi: 10.1016/0076-6879(87)52054-7. [DOI] [PubMed] [Google Scholar]
- Myers A. M., Tzagoloff A. MSW, a yeast gene coding for mitochondrial tryptophanyl-tRNA synthetase. J Biol Chem. 1985 Dec 5;260(28):15371–15377. [PubMed] [Google Scholar]
- Rould M. A., Perona J. J., Söll D., Steitz T. A. Structure of E. coli glutaminyl-tRNA synthetase complexed with tRNA(Gln) and ATP at 2.8 A resolution. Science. 1989 Dec 1;246(4934):1135–1142. doi: 10.1126/science.2479982. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scolnick E. M., Caskey C. T. Peptide chain termination. V. The role of release factors in mRNA terminator codon recognition. Proc Natl Acad Sci U S A. 1969 Dec;64(4):1235–1241. doi: 10.1073/pnas.64.4.1235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scolnick E., Tompkins R., Caskey T., Nirenberg M. Release factors differing in specificity for terminator codons. Proc Natl Acad Sci U S A. 1968 Oct;61(2):768–774. doi: 10.1073/pnas.61.2.768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Short J. M., Fernandez J. M., Sorge J. A., Huse W. D. Lambda ZAP: a bacteriophage lambda expression vector with in vivo excision properties. Nucleic Acids Res. 1988 Aug 11;16(15):7583–7600. doi: 10.1093/nar/16.15.7583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith L. M., Sanders J. Z., Kaiser R. J., Hughes P., Dodd C., Connell C. R., Heiner C., Kent S. B., Hood L. E. Fluorescence detection in automated DNA sequence analysis. Nature. 1986 Jun 12;321(6071):674–679. doi: 10.1038/321674a0. [DOI] [PubMed] [Google Scholar]
- Tate W. P., Beaudet A. L., Caskey C. T. Influence of guanine nucleotides and elongation factors on interaction of release factors with the ribosome. Proc Natl Acad Sci U S A. 1973 Aug;70(8):2350–2355. doi: 10.1073/pnas.70.8.2350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tate W. P., Caskey C. T. The mechanism of peptide chain termination. Mol Cell Biochem. 1974 Dec 20;5(3):115–126. doi: 10.1007/BF01731375. [DOI] [PubMed] [Google Scholar]
- Wong G. G., Witek J. S., Temple P. A., Wilkens K. M., Leary A. C., Luxenberg D. P., Jones S. S., Brown E. L., Kay R. M., Orr E. C. Human GM-CSF: molecular cloning of the complementary DNA and purification of the natural and recombinant proteins. Science. 1985 May 17;228(4701):810–815. doi: 10.1126/science.3923623. [DOI] [PubMed] [Google Scholar]
- de Duve C. Transfer RNAs: the second genetic code. Nature. 1988 May 12;333(6169):117–118. doi: 10.1038/333117a0. [DOI] [PubMed] [Google Scholar]