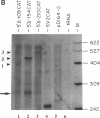
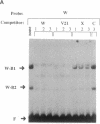
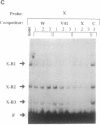
Abstract
Previous reports have identified that the class II box, consisting of the positive regulatory X and Y boxes, is important for expression of all class II major histocompatibility genes. In this paper, we identify additional sequences upstream from the class II box that regulate constitutive transcription of a human class II gene, HLA-DRA, in the B-lymphoblastoid cell line Raji. Using 5' promoter deletions, substitution mutants, and nuclease S1 protection assays, we mapped a positive element, called W, between -135 and -117 base pairs and a negative element, called V, from -193 to -179 base pairs. Sequence comparisons revealed that W and V share homology with the HLA-DRA X box situated downstream. Gel-mobility-shift assays confirmed that the Raji nuclear proteins that bound to W and V elements were competed with by an HLA-DRA X-box oligonucleotide. These results suggest that X-box-binding proteins mediate both positive and negative effects on transcription by means of interaction with multiple elements (W, V, and X) within the same HLA-DRA gene.
Full text
PDF

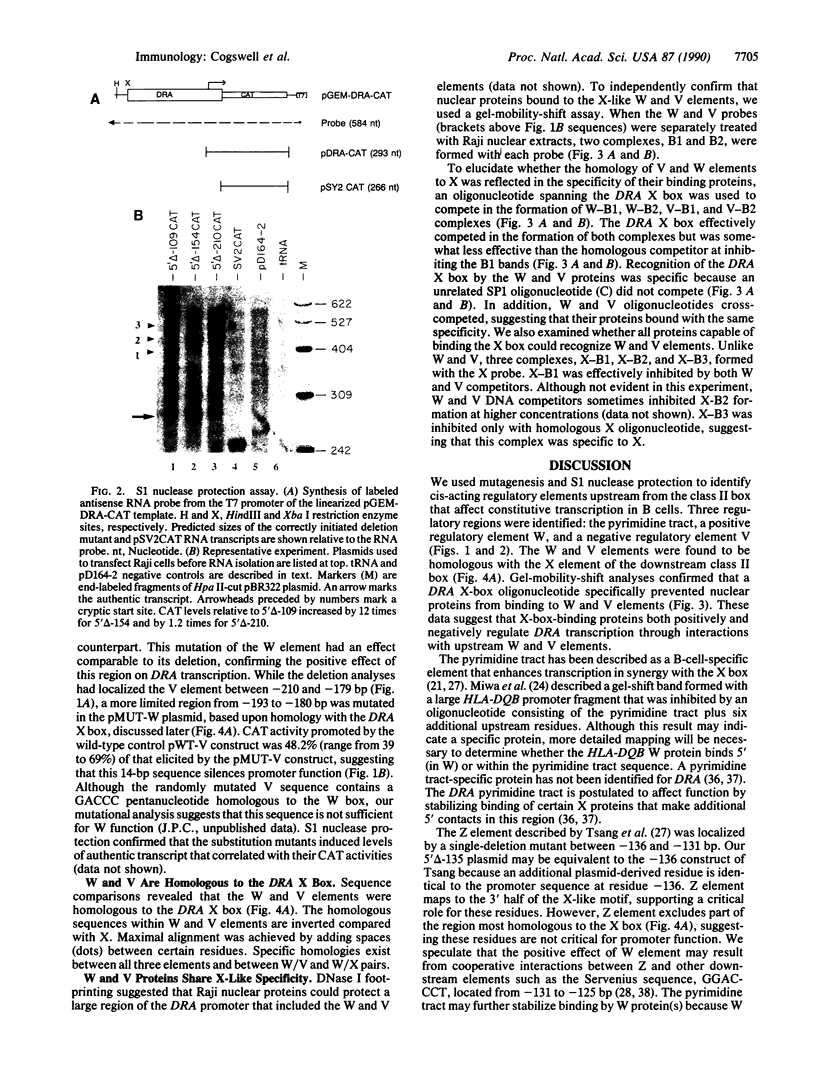

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Babbitt B. P., Allen P. M., Matsueda G., Haber E., Unanue E. R. Binding of immunogenic peptides to Ia histocompatibility molecules. 1985 Sep 26-Oct 2Nature. 317(6035):359–361. doi: 10.1038/317359a0. [DOI] [PubMed] [Google Scholar]
- Barberis A., Superti-Furga G., Busslinger M. Mutually exclusive interaction of the CCAAT-binding factor and of a displacement protein with overlapping sequences of a histone gene promoter. Cell. 1987 Jul 31;50(3):347–359. doi: 10.1016/0092-8674(87)90489-2. [DOI] [PubMed] [Google Scholar]
- Basta P. V., Sherman P. A., Ting J. P. Detailed delineation of an interferon-gamma-responsive element important in human HLA-DRA gene expression in a glioblastoma multiform line. Proc Natl Acad Sci U S A. 1988 Nov;85(22):8618–8622. doi: 10.1073/pnas.85.22.8618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basta P. V., Sherman P. A., Ting J. P. Identification of an interferon-gamma response region 5' of the human histocompatibility leukocyte antigen DR alpha chain gene which is active in human glioblastoma multiforme lines. J Immunol. 1987 Feb 15;138(4):1275–1280. [PubMed] [Google Scholar]
- Berk A. J., Sharp P. A. Sizing and mapping of early adenovirus mRNAs by gel electrophoresis of S1 endonuclease-digested hybrids. Cell. 1977 Nov;12(3):721–732. doi: 10.1016/0092-8674(77)90272-0. [DOI] [PubMed] [Google Scholar]
- Boothby M., Liou H. C., Glimcher L. H. Differences in DNA sequence specificity among MHC class II X box binding proteins. J Immunol. 1989 Feb 1;142(3):1005–1014. [PubMed] [Google Scholar]
- Calman A. F., Peterlin B. M. Evidence for a trans-acting factor that regulates the transcription of class II major histocompatibility complex genes: genetic and functional analysis. Proc Natl Acad Sci U S A. 1988 Dec;85(23):8830–8834. doi: 10.1073/pnas.85.23.8830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Celada A., Maki R. Evidence for multiple major histocompatibility class II X-box binding proteins. Mol Cell Biol. 1989 Nov;9(11):5219–5222. doi: 10.1128/mcb.9.11.5219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Cogswell J. P., Phipps R. P., Scott D. W. Role of self carriers in the immune response and tolerance. XI. Correlation of Ia expression and interleukin-1 production with delivery of immunogenic signals in vivo by hapten-modified accessory cell-like tumor lines. Cell Immunol. 1988 Jun;114(1):55–70. doi: 10.1016/0008-8749(88)90254-7. [DOI] [PubMed] [Google Scholar]
- Dignam J. D., Lebovitz R. M., Roeder R. G. Accurate transcription initiation by RNA polymerase II in a soluble extract from isolated mammalian nuclei. Nucleic Acids Res. 1983 Mar 11;11(5):1475–1489. doi: 10.1093/nar/11.5.1475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorn A., Bollekens J., Staub A., Benoist C., Mathis D. A multiplicity of CCAAT box-binding proteins. Cell. 1987 Sep 11;50(6):863–872. doi: 10.1016/0092-8674(87)90513-7. [DOI] [PubMed] [Google Scholar]
- Dorn A., Durand B., Marfing C., Le Meur M., Benoist C., Mathis D. Conserved major histocompatibility complex class II boxes--X and Y--are transcriptional control elements and specifically bind nuclear proteins. Proc Natl Acad Sci U S A. 1987 Sep;84(17):6249–6253. doi: 10.1073/pnas.84.17.6249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorn A., Fehling H. J., Koch W., Le Meur M., Gerlinger P., Benoist C., Mathis D. B-cell control region at the 5' end of a major histocompatibility complex class II gene: sequences and factors. Mol Cell Biol. 1988 Oct;8(10):3975–3987. doi: 10.1128/mcb.8.10.3975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glass C. K., Lipkin S. M., Devary O. V., Rosenfeld M. G. Positive and negative regulation of gene transcription by a retinoic acid-thyroid hormone receptor heterodimer. Cell. 1989 Nov 17;59(4):697–708. doi: 10.1016/0092-8674(89)90016-0. [DOI] [PubMed] [Google Scholar]
- Gorman C. M., Moffat L. F., Howard B. H. Recombinant genomes which express chloramphenicol acetyltransferase in mammalian cells. Mol Cell Biol. 1982 Sep;2(9):1044–1051. doi: 10.1128/mcb.2.9.1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gönczy P., Reith W., Barras E., Lisowska-Grospierre B., Griscelli C., Hadam M. R., Mach B. Inherited immunodeficiency with a defect in a major histocompatibility complex class II promoter-binding protein differs in the chromatin structure of the HLA-DRA gene. Mol Cell Biol. 1989 Jan;9(1):296–302. doi: 10.1128/mcb.9.1.296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hai T. W., Liu F., Coukos W. J., Green M. R. Transcription factor ATF cDNA clones: an extensive family of leucine zipper proteins able to selectively form DNA-binding heterodimers. Genes Dev. 1989 Dec;3(12B):2083–2090. doi: 10.1101/gad.3.12b.2083. [DOI] [PubMed] [Google Scholar]
- Heber-Katz E., Hansburg D., Schwartz R. H. The Ia molecule of the antigen-presenting cell plays a critical role in immune response gene regulation of T cell activation. J Mol Cell Immunol. 1983;1(1):3–18. [PubMed] [Google Scholar]
- Hood L., Steinmetz M., Malissen B. Genes of the major histocompatibility complex of the mouse. Annu Rev Immunol. 1983;1:529–568. doi: 10.1146/annurev.iy.01.040183.002525. [DOI] [PubMed] [Google Scholar]
- Kaufman J. F., Auffray C., Korman A. J., Shackelford D. A., Strominger J. The class II molecules of the human and murine major histocompatibility complex. Cell. 1984 Jan;36(1):1–13. doi: 10.1016/0092-8674(84)90068-0. [DOI] [PubMed] [Google Scholar]
- Kelly A., Trowsdale J. Complete nucleotide sequence of a functional HLA-DP beta gene and the region between the DP beta 1 and DP alpha 1 genes: comparison of the 5' ends of HLA class II genes. Nucleic Acids Res. 1985 Mar 11;13(5):1607–1621. doi: 10.1093/nar/13.5.1607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lechler R. I., Norcross M. A., Germain R. N. Qualitative and quantitative studies of antigen-presenting cell function by using I-A-expressing L cells. J Immunol. 1985 Nov;135(5):2914–2922. [PubMed] [Google Scholar]
- Levine M., Manley J. L. Transcriptional repression of eukaryotic promoters. Cell. 1989 Nov 3;59(3):405–408. doi: 10.1016/0092-8674(89)90024-x. [DOI] [PubMed] [Google Scholar]
- Liou H. C., Boothby M. R., Glimcher L. H. Distinct cloned class II MHC DNA binding proteins recognize the X box transcription element. Science. 1988 Oct 7;242(4875):69–71. doi: 10.1126/science.3140376. [DOI] [PubMed] [Google Scholar]
- Maniatis T., Goodbourn S., Fischer J. A. Regulation of inducible and tissue-specific gene expression. Science. 1987 Jun 5;236(4806):1237–1245. doi: 10.1126/science.3296191. [DOI] [PubMed] [Google Scholar]
- Mathis D. J., Benoist C. O., Williams V. E., 2nd, Kanter M. R., McDevitt H. O. The murine E alpha immune response gene. Cell. 1983 Mar;32(3):745–754. doi: 10.1016/0092-8674(83)90060-0. [DOI] [PubMed] [Google Scholar]
- Mattaj I. W., Lienhard S., Jiricny J., De Robertis E. M. An enhancer-like sequence within the Xenopus U2 gene promoter facilitates the formation of stable transcription complexes. Nature. 1985 Jul 11;316(6024):163–167. doi: 10.1038/316163a0. [DOI] [PubMed] [Google Scholar]
- Melton D. A., Krieg P. A., Rebagliati M. R., Maniatis T., Zinn K., Green M. R. Efficient in vitro synthesis of biologically active RNA and RNA hybridization probes from plasmids containing a bacteriophage SP6 promoter. Nucleic Acids Res. 1984 Sep 25;12(18):7035–7056. doi: 10.1093/nar/12.18.7035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miwa K., Doyle C., Strominger J. L. Sequence-specific interactions of nuclear factors with conserved sequences of human class II major histocompatibility complex genes. Proc Natl Acad Sci U S A. 1987 Jul;84(14):4939–4943. doi: 10.1073/pnas.84.14.4939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oro A. E., Hollenberg S. M., Evans R. M. Transcriptional inhibition by a glucocorticoid receptor-beta-galactosidase fusion protein. Cell. 1988 Dec 23;55(6):1109–1114. doi: 10.1016/0092-8674(88)90255-3. [DOI] [PubMed] [Google Scholar]
- Parslow T. G., Blair D. L., Murphy W. J., Granner D. K. Structure of the 5' ends of immunoglobulin genes: a novel conserved sequence. Proc Natl Acad Sci U S A. 1984 May;81(9):2650–2654. doi: 10.1073/pnas.81.9.2650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poellinger L., Yoza B. K., Roeder R. G. Functional cooperativity between protein molecules bound at two distinct sequence elements of the immunoglobulin heavy-chain promoter. Nature. 1989 Feb 9;337(6207):573–576. doi: 10.1038/337573a0. [DOI] [PubMed] [Google Scholar]
- Reith W., Barras E., Satola S., Kobr M., Reinhart D., Sanchez C. H., Mach B. Cloning of the major histocompatibility complex class II promoter binding protein affected in a hereditary defect in class II gene regulation. Proc Natl Acad Sci U S A. 1989 Jun;86(11):4200–4204. doi: 10.1073/pnas.86.11.4200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reith W., Satola S., Sanchez C. H., Amaldi I., Lisowska-Grospierre B., Griscelli C., Hadam M. R., Mach B. Congenital immunodeficiency with a regulatory defect in MHC class II gene expression lacks a specific HLA-DR promoter binding protein, RF-X. Cell. 1988 Jun 17;53(6):897–906. doi: 10.1016/s0092-8674(88)90389-3. [DOI] [PubMed] [Google Scholar]
- Sakurai M., Strominger J. L. B-cell-specific enhancer activity of conserved upstream elements of the class II major histocompatibility complex DQB gene. Proc Natl Acad Sci U S A. 1988 Sep;85(18):6909–6913. doi: 10.1073/pnas.85.18.6909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Servenius B., Rask L., Peterson P. A. Class II genes of the human major histocompatibility complex. The DO beta gene is a divergent member of the class II beta gene family. J Biol Chem. 1987 Jun 25;262(18):8759–8766. [PubMed] [Google Scholar]
- Sherman P. A., Basta P. V., Heguy A., Wloch M. K., Roeder R. G., Ting J. P. The octamer motif is a B-lymphocyte-specific regulatory element of the HLA-DR alpha gene promoter. Proc Natl Acad Sci U S A. 1989 Sep;86(17):6739–6743. doi: 10.1073/pnas.86.17.6739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherman P. A., Basta P. V., Moore T. L., Brown A. M., Ting J. P. Class II box consensus sequences in the HLA-DR alpha gene: transcriptional function and interaction with nuclear proteins. Mol Cell Biol. 1989 Jan;9(1):50–56. doi: 10.1128/mcb.9.1.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherman P. A., Basta P. V., Ting J. P. Upstream DNA sequences required for tissue-specific expression of the HLA-DR alpha gene. Proc Natl Acad Sci U S A. 1987 Jun;84(12):4254–4258. doi: 10.1073/pnas.84.12.4254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh H., Sen R., Baltimore D., Sharp P. A. A nuclear factor that binds to a conserved sequence motif in transcriptional control elements of immunoglobulin genes. Nature. 1986 Jan 9;319(6049):154–158. doi: 10.1038/319154a0. [DOI] [PubMed] [Google Scholar]
- Sive H. L., Roeder R. G. Interaction of a common factor with conserved promoter and enhancer sequences in histone H2B, immunoglobulin, and U2 small nuclear RNA (snRNA) genes. Proc Natl Acad Sci U S A. 1986 Sep;83(17):6382–6386. doi: 10.1073/pnas.83.17.6382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsang S. Y., Nakanishi M., Peterlin B. M. B-cell-specific and interferon-gamma-inducible regulation of the HLA-DR alpha gene. Proc Natl Acad Sci U S A. 1988 Nov;85(22):8598–8602. doi: 10.1073/pnas.85.22.8598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unanue E. R., Beller D. I., Lu C. Y., Allen P. M. Antigen presentation: comments on its regulation and mechanism. J Immunol. 1984 Jan;132(1):1–5. [PubMed] [Google Scholar]
- Unanue E. R. The regulatory role of macrophages in antigenic stimulation. Part Two: symbiotic relationship between lymphocytes and macrophages. Adv Immunol. 1981;31:1–136. doi: 10.1016/s0065-2776(08)60919-0. [DOI] [PubMed] [Google Scholar]
- Zinn K., Maniatis T. Detection of factors that interact with the human beta-interferon regulatory region in vivo by DNAase I footprinting. Cell. 1986 May 23;45(4):611–618. doi: 10.1016/0092-8674(86)90293-x. [DOI] [PubMed] [Google Scholar]