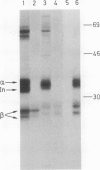
Abstract
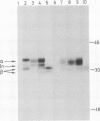
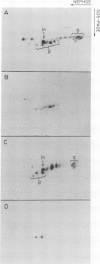
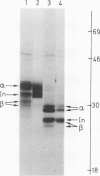
HLA-DR antigens are polymorphic cell surface glycoproteins, expressed primarily in B lymphocytes and macrophages, which are thought to play an important role in the immune response. Two polypeptide chains, alpha and beta, are associated at the cell surface, and a third chain associates with alpha and beta intracellularly. RNA isolated from the human B-cell line Raji was injected in Xenopus laevis oocytes. Immunoprecipitates of translation products with several monoclonal antibodies revealed the presence of HLA-DR antigens similar to those synthesized in Raji cells. One monoclonal antibody was able to bind the beta chain after dissociation of the three polypeptide chains with detergent. The presence of all three chains was confirmed by two-dimensional gel electrophoresis. The glycosylation pattern of the three chains was identical to that observed in vivo, as evidenced in studies using tunicamycin, an inhibitor of N-linked glycosylation. The presence of alpha chains assembled with beta chains in equimolar ratio was further demonstrated by amino-terminal sequencing. An RNA fraction enriched for the three mRNAs, encoding alpha, beta, and intracellular chains, was isolated. This translation-assembly system and the availability of monoclonal antibodies make it possible to assay for mRNA encoding specific molecules among the multiple human Ia-like antigens.
Full text
PDF
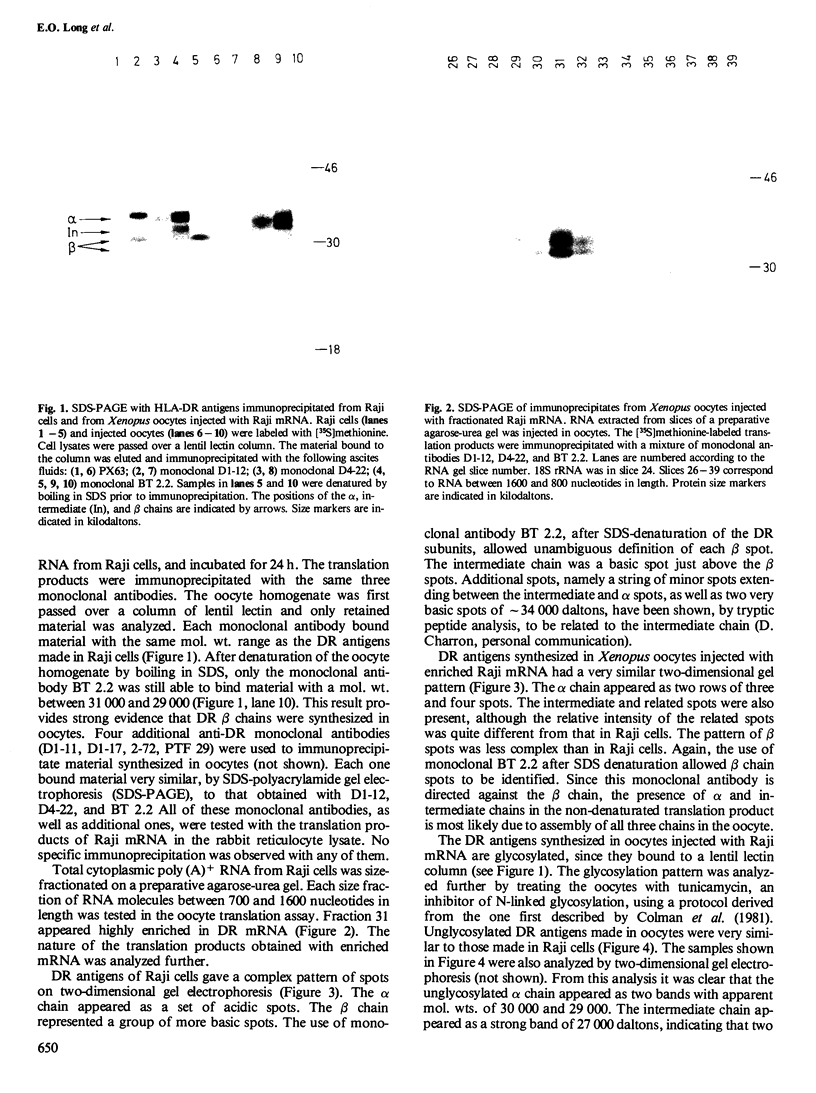
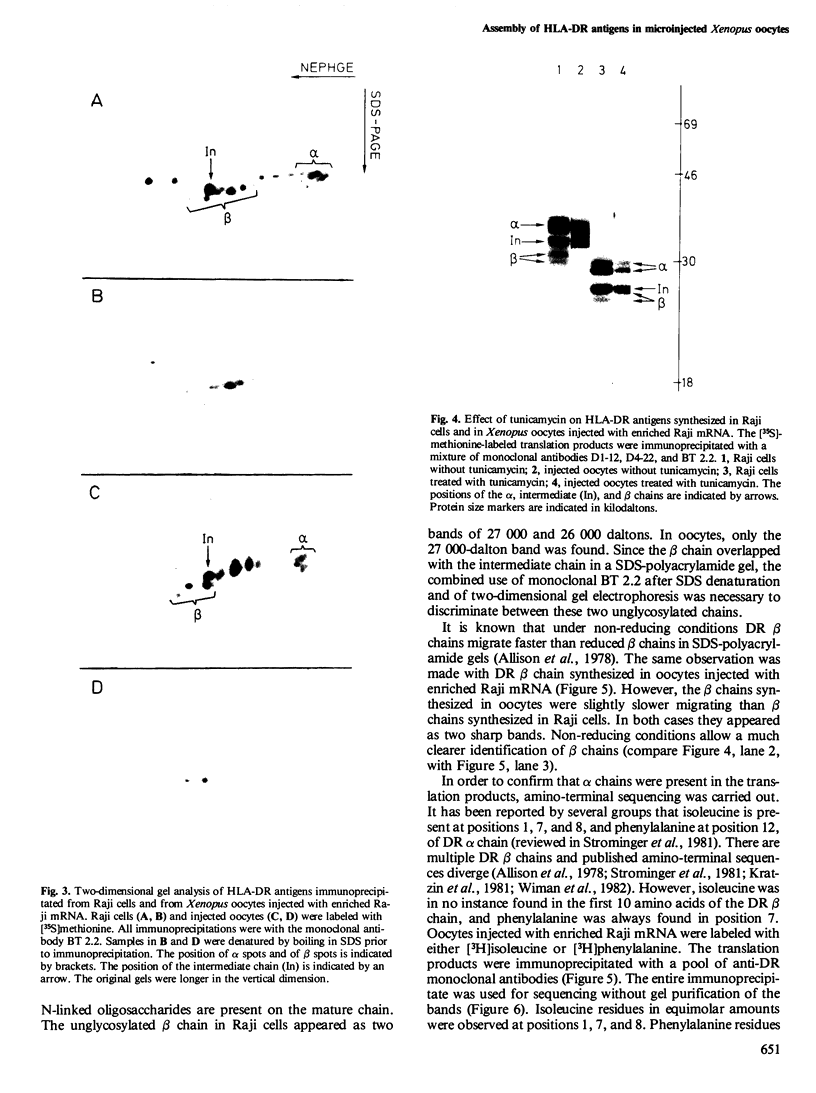
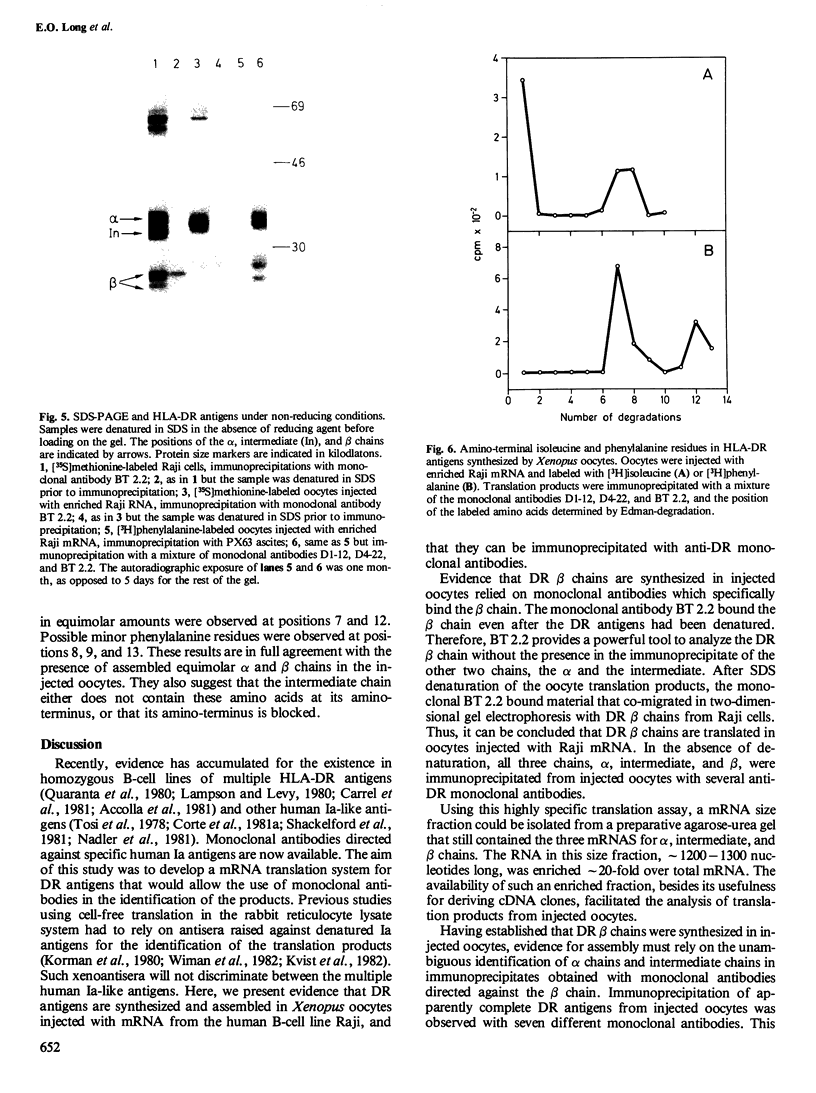


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Accolla R. S., Gross N., Carrel S., Corte G. Distinct forms of both alpha and beta subunits are present in the human Ia molecular pool. Proc Natl Acad Sci U S A. 1981 Jul;78(7):4549–4551. doi: 10.1073/pnas.78.7.4549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Accolla R. S., Sekaly R. P., McDonald A. P., Corte G., Gross N., Carrel S. Demonstration at the single-cell level of the existence of distinct clusters of epitopes in two predefined human Ia molecular subsets. Eur J Immunol. 1982 Feb;12(2):166–169. doi: 10.1002/eji.1830120212. [DOI] [PubMed] [Google Scholar]
- Allison J. P., Walker L. E., Russell W. A., Pellegrino M. A., Ferrone S., Reisfeld R. A., Frelinger J. A., Silver J. Murine Ia and human DR antigens: homology of amino-terminal sequences. Proc Natl Acad Sci U S A. 1978 Aug;75(8):3953–3956. doi: 10.1073/pnas.75.8.3953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asselbergs F. A. Post-synthetic fate of the translation products of messenger RNA microinjected into Xenopus oocytes. Mol Biol Rep. 1979 Dec 31;5(4):199–208. doi: 10.1007/BF00782889. [DOI] [PubMed] [Google Scholar]
- Bach F. H., van Rood J. J. The major histocompatibility complex--genetics and biology. (First of three parts). N Engl J Med. 1976 Oct 7;295(15):806–813. doi: 10.1056/NEJM197610072951504. [DOI] [PubMed] [Google Scholar]
- Carrel S., Tosi R., Gross N., Tanigaki N., Carmagnola A. L., Accolla R. S. Subsets of human Ia-like molecules defined by monoclonal antibodies. Mol Immunol. 1981 May;18(5):403–411. doi: 10.1016/0161-5890(81)90102-4. [DOI] [PubMed] [Google Scholar]
- Charron D. J., McDevitt H. O. Analysis of HLA-D region-associated molecules with monoclonal antibody. Proc Natl Acad Sci U S A. 1979 Dec;76(12):6567–6571. doi: 10.1073/pnas.76.12.6567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charron D. J., McDevitt H. O. Characterization of HLA-D-region antigens by two-dimensional gel electrophoresis. Molecular-genotyping. J Exp Med. 1980 Aug 1;152(2 Pt 2):18s–36s. [PubMed] [Google Scholar]
- Colman A., Lane C. D., Craig R., Boulton A., Mohun T., Morser J. The influence of topology and glycosylation on the fate of heterologous secretory proteins made in Xenopus oocytes. Eur J Biochem. 1981 Jan;113(2):339–348. doi: 10.1111/j.1432-1033.1981.tb05072.x. [DOI] [PubMed] [Google Scholar]
- Corte G., Calabi F., Damiani G., Bargellesi A., Tosi R., Sorrentino R. Human Ia molecules carrying DC1 determinants differ in both alpha- and beta-subunits from Ia molecules carrying DR determinants. Nature. 1981 Jul 23;292(5821):357–360. doi: 10.1038/292357a0. [DOI] [PubMed] [Google Scholar]
- Finn O. J., Levy R. Multiple HLA-DR antigens: detection with monoclonal antibodies and translation in vitro. Proc Natl Acad Sci U S A. 1982 Apr;79(8):2658–2662. doi: 10.1073/pnas.79.8.2658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones P. P., Murphy D. B., Hewgill D., McDevitt H. O. Detection of a common polypeptide chain in I--A and I--E sub-region immunoprecipitates. Mol Immunol. 1979 Jan;16(1):51–60. doi: 10.1016/0161-5890(79)90027-0. [DOI] [PubMed] [Google Scholar]
- Klein J. The major histocompatibility complex of the mouse. Science. 1979 Feb 9;203(4380):516–521. doi: 10.1126/science.104386. [DOI] [PubMed] [Google Scholar]
- Korman A. J., Ploegh H. L., Kaufman J. F., Owen M. J., Strominger J. L. Cell-free synthesis and processing of the heavy and light chains of HLA-DR antigens. J Exp Med. 1980 Aug 1;152(2 Pt 2):65s–82s. [PubMed] [Google Scholar]
- Kratzin H., Yang C. Y., Götz H., Pauly E., Kölbel S., Egert G., Thinnes F. P., Wernet P., Altevogt P., Hilschmann N. Primärstruktur menschlicher Histokompatibilitätsantigene der Klasse II. 1. Mitteilung: Aminosäuresequenz der N-terminalen 198 Reste der beta-Kette des HLA-Dw2,2;DR2,2-Alloantigens. Hoppe Seylers Z Physiol Chem. 1981 Dec;362(12):1665–1669. [PubMed] [Google Scholar]
- Lampson L. A., Levy R. Two populations of Ia-like molecules on a human B cell line. J Immunol. 1980 Jul;125(1):293–299. [PubMed] [Google Scholar]
- Lehrach H., Diamond D., Wozney J. M., Boedtker H. RNA molecular weight determinations by gel electrophoresis under denaturing conditions, a critical reexamination. Biochemistry. 1977 Oct 18;16(21):4743–4751. doi: 10.1021/bi00640a033. [DOI] [PubMed] [Google Scholar]
- McMillan M., Frelinger J. A., Jones P. P., Murphy D. B., McDevitt H. O., Hood L. Structure of murine Ia antigens. Two dimensional electrophoretic analyses and high pressure liquid chromatography tryptic peptide maps of products of the I-A and I-E subregions and of an associated invariant polypeptide. J Exp Med. 1981 Apr 1;153(4):936–950. doi: 10.1084/jem.153.4.936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moar V. A., Gurdon J. B., Lane C. D., Marbaix G. Translational capacity of living frog eggs and oocytes, as judged by messenger RNA injection. J Mol Biol. 1971 Oct 14;61(1):93–103. doi: 10.1016/0022-2836(71)90208-7. [DOI] [PubMed] [Google Scholar]
- Nadler L. M., Stashenko P., Hardy R., Tomaselli K. J., Yunis E. J., Schlossman S. F., Pesando J. M. Monoclonal antibody identifies a new Ia-like (p29,34) polymorphic system linked to the HLA-D/DR region. Nature. 1981 Apr 16;290(5807):591–593. doi: 10.1038/290591a0. [DOI] [PubMed] [Google Scholar]
- O'Farrell P. Z., Goodman H. M., O'Farrell P. H. High resolution two-dimensional electrophoresis of basic as well as acidic proteins. Cell. 1977 Dec;12(4):1133–1141. doi: 10.1016/0092-8674(77)90176-3. [DOI] [PubMed] [Google Scholar]
- Owen M. J., Kissonerghis A. M., Lodish H. F., Crumpton M. J. Biosynthesis and maturation of HLA-DR antigens in vivo. J Biol Chem. 1981 Sep 10;256(17):8987–8993. [PubMed] [Google Scholar]
- Quaranta V., Walker L. E., Pellegrino M. A., Ferrone S. Purification of immunologically functional subsets of human Ia-like antigens on a monoclonal antibody (Q5/13) immunoadsorbent. J Immunol. 1980 Oct;125(4):1421–1425. [PubMed] [Google Scholar]
- Rosen J. M., Woo S. L., Holder J. W., Means A. R., O'Malley B. W. Preparation and preliminary characterization of purified ovalbumin messenger RNA from the hen oviduct. Biochemistry. 1975 Jan 14;14(1):69–78. doi: 10.1021/bi00672a012. [DOI] [PubMed] [Google Scholar]
- Rungger D., Türler H. DNAs of simian virus 40 and polyoma direct the synthesis of viral tumor antigens and capsid proteins in Xenopus oocytes. Proc Natl Acad Sci U S A. 1978 Dec;75(12):6073–6077. doi: 10.1073/pnas.75.12.6073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryder L. P., Svejgaard A., Dausset J. Genetics of HLA disease association. Annu Rev Genet. 1981;15:169–187. doi: 10.1146/annurev.ge.15.120181.001125. [DOI] [PubMed] [Google Scholar]
- Shackelford D. A., Mann D. L., van Rood J. J., Ferrara G. B., Strominger J. L. Human B-cell alloantigens DC1, MT1, and LB12 are identical to each other but distinct from the HLA-DR antigen. Proc Natl Acad Sci U S A. 1981 Jul;78(7):4566–4570. doi: 10.1073/pnas.78.7.4566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shackelford D. A., Strominger J. L. Demonstration of structural polymorphism among HLA-DR light chains by two-dimensional gel electrophoresis. J Exp Med. 1980 Jan 1;151(1):144–165. doi: 10.1084/jem.151.1.144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silver J., Ferrone S. Structural polymorphism of human DR antigens. Nature. 1979 May 31;279(5712):436–437. doi: 10.1038/279436a0. [DOI] [PubMed] [Google Scholar]
- Tosi R., Tanigaki N., Centis D., Ferrara G. B., Pressman D. Immunological dissection of human Ia molecules. J Exp Med. 1978 Dec 1;148(6):1592–1611. doi: 10.1084/jem.148.6.1592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker L. E., Ferrone S., Pellegrino M. A., Reisfeld R. A. Structural polymorphism of the beta chain of human HLA-DR antigens. Mol Immunol. 1980 Dec;17(12):1443–1448. doi: 10.1016/0161-5890(80)90169-8. [DOI] [PubMed] [Google Scholar]
- Wallace R. A., Jared D. W., Dumont J. N., Sega M. W. Protein incorporation by isolated amphibian oocytes. 3. Optimum incubation conditions. J Exp Zool. 1973 Jun;184(3):321–333. doi: 10.1002/jez.1401840305. [DOI] [PubMed] [Google Scholar]
- Wiman K., Larhammar D., Claesson L., Gustafsson K., Schenning L., Bill P., Böhme J., Denaro M., Dobberstein B., Hammerling U. Isolation and identification of a cDNA clone corresponding to an HLA-DR antigen beta chain. Proc Natl Acad Sci U S A. 1982 Mar;79(6):1703–1707. doi: 10.1073/pnas.79.6.1703. [DOI] [PMC free article] [PubMed] [Google Scholar]