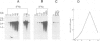Abstract
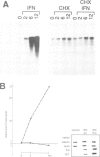
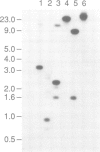
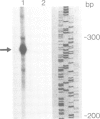
Here we report the molecular cloning of several related human cDNAs from which a full-length sequence can be determined. The cDNAs encode a 2.8 kb mRNA that is strongly induced by interferon (IFN) gamma and the expression of which is not cell-restricted but observed in fibroblasts, macrophages and epithelial cells. The deduced amino acid sequence predicts a protein of 471 amino acids with high sequence similarity to a previously identified rabbit peptide chain release factor. Functional studies to demonstrate release factor activity showed that the protein encoded by this cDNA inhibited the readthrough activity of a yeast UGA suppressor tRNA in an in vitro translation system. The identification of this novel cDNA implies that translational control by IFN induced proteins may not be restricted to the initial steps of protein synthesis but may also act by regulation of peptide chain termination.
Full text
PDF
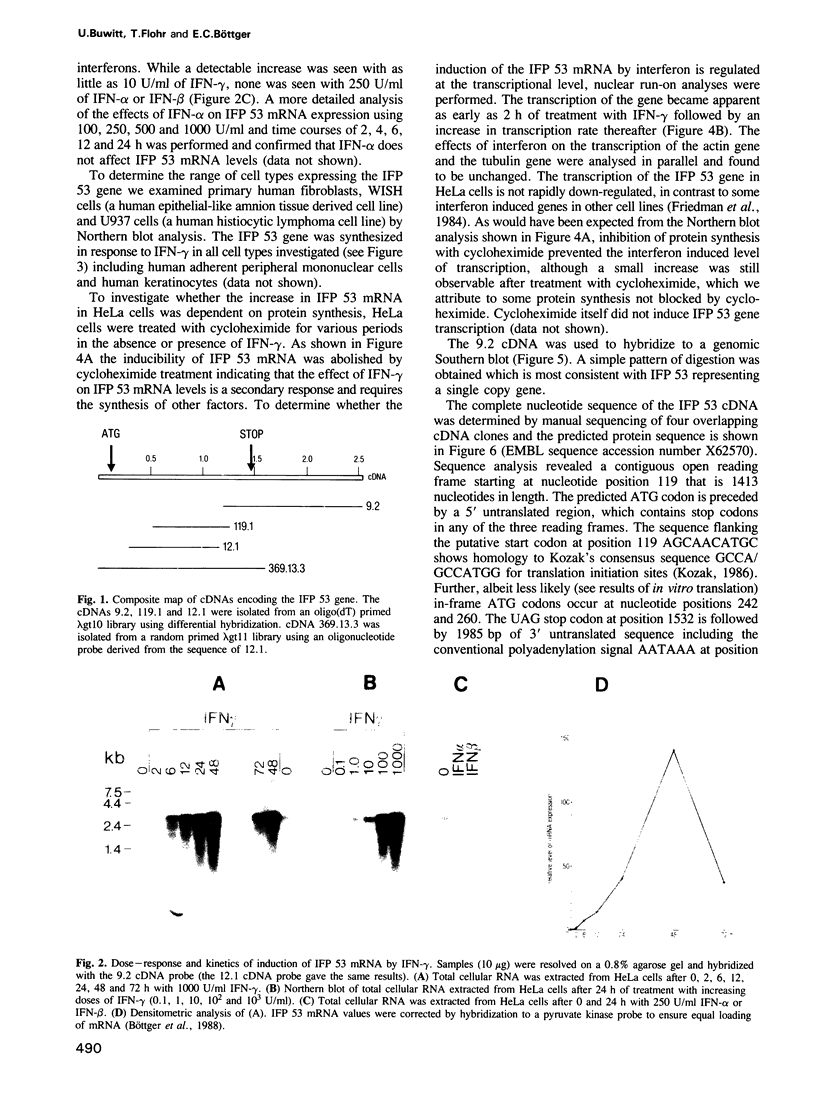




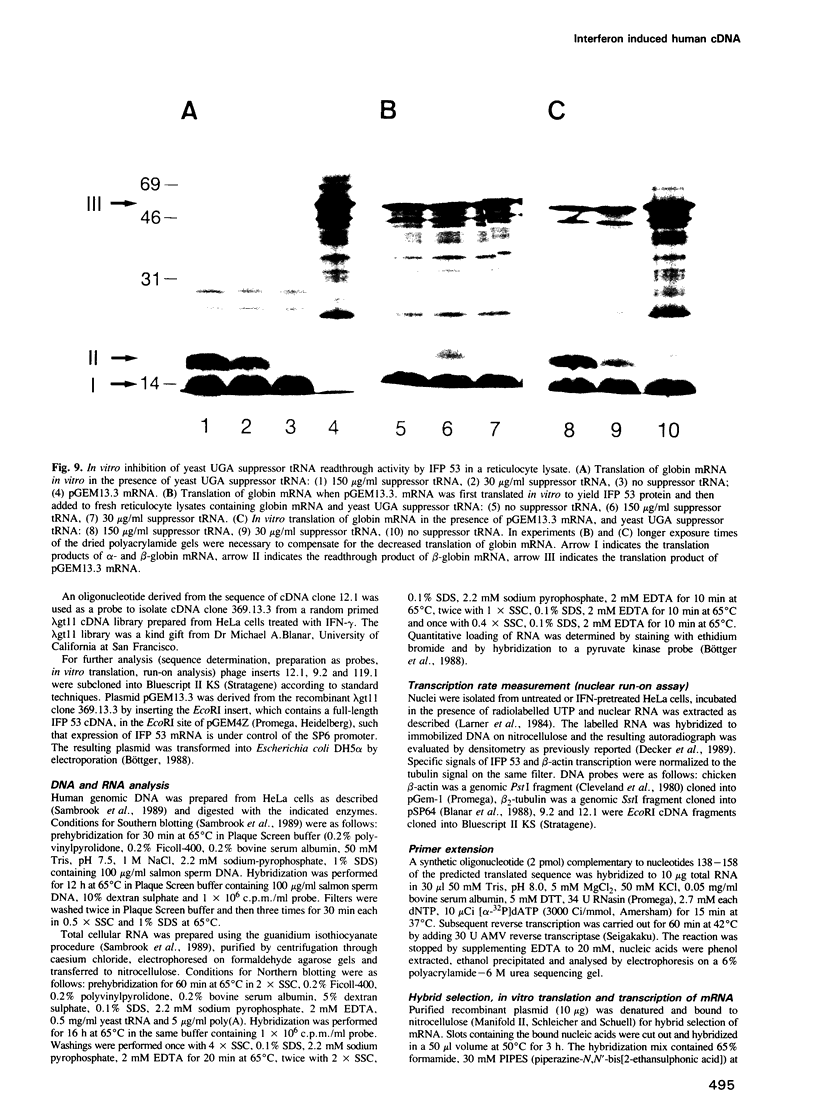

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andreason G. L., Evans G. A. Introduction and expression of DNA molecules in eukaryotic cells by electroporation. Biotechniques. 1988 Jul-Aug;6(7):650–660. [PubMed] [Google Scholar]
- Arnheiter H., Haller O. Antiviral state against influenza virus neutralized by microinjection of antibodies to interferon-induced Mx proteins. EMBO J. 1988 May;7(5):1315–1320. doi: 10.1002/j.1460-2075.1988.tb02946.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benech P., Mory Y., Revel M., Chebath J. Structure of two forms of the interferon-induced (2'-5') oligo A synthetase of human cells based on cDNAs and gene sequences. EMBO J. 1985 Sep;4(9):2249–2256. doi: 10.1002/j.1460-2075.1985.tb03922.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanar M. A., Boettger E. C., Flavell R. A. Transcriptional activation of HLA-DR alpha by interferon gamma requires a trans-acting protein. Proc Natl Acad Sci U S A. 1988 Jul;85(13):4672–4676. doi: 10.1073/pnas.85.13.4672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Böck A., Forchhammer K., Heider J., Leinfelder W., Sawers G., Veprek B., Zinoni F. Selenocysteine: the 21st amino acid. Mol Microbiol. 1991 Mar;5(3):515–520. doi: 10.1111/j.1365-2958.1991.tb00722.x. [DOI] [PubMed] [Google Scholar]
- Böttger E. C., Blanar M. A., Flavell R. A. Cycloheximide, an inhibitor of protein synthesis, prevents gamma-interferon-induced expression of class II mRNA in a macrophage cell line. Immunogenetics. 1988;28(4):215–220. doi: 10.1007/BF00345497. [DOI] [PubMed] [Google Scholar]
- Caskey C. T., Beaudet A. L., Tate W. P. Mammalian release factor; in vitro assay and purification. Methods Enzymol. 1974;30:293–303. doi: 10.1016/0076-6879(74)30032-8. [DOI] [PubMed] [Google Scholar]
- Cleveland D. W., Lopata M. A., MacDonald R. J., Cowan N. J., Rutter W. J., Kirschner M. W. Number and evolutionary conservation of alpha- and beta-tubulin and cytoplasmic beta- and gamma-actin genes using specific cloned cDNA probes. Cell. 1980 May;20(1):95–105. doi: 10.1016/0092-8674(80)90238-x. [DOI] [PubMed] [Google Scholar]
- Craigen W. J., Caskey C. T. The function, structure and regulation of E. coli peptide chain release factors. Biochimie. 1987 Oct;69(10):1031–1041. doi: 10.1016/0300-9084(87)90003-4. [DOI] [PubMed] [Google Scholar]
- Craigen W. J., Lee C. C., Caskey C. T. Recent advances in peptide chain termination. Mol Microbiol. 1990 Jun;4(6):861–865. doi: 10.1111/j.1365-2958.1990.tb00658.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Decker T., Lew D. J., Cheng Y. S., Levy D. E., Darnell J. E., Jr Interactions of alpha- and gamma-interferon in the transcriptional regulation of the gene encoding a guanylate-binding protein. EMBO J. 1989 Jul;8(7):2009–2014. doi: 10.1002/j.1460-2075.1989.tb03608.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Efstratiadis A., Kafatos F. C., Maniatis T. The primary structure of rabbit beta-globin mRNA as determined from cloned DNA. Cell. 1977 Apr;10(4):571–585. doi: 10.1016/0092-8674(77)90090-3. [DOI] [PubMed] [Google Scholar]
- Farber J. M. A macrophage mRNA selectively induced by gamma-interferon encodes a member of the platelet factor 4 family of cytokines. Proc Natl Acad Sci U S A. 1990 Jul;87(14):5238–5242. doi: 10.1073/pnas.87.14.5238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farrell P. J., Balkow K., Hunt T., Jackson R. J., Trachsel H. Phosphorylation of initiation factor elF-2 and the control of reticulocyte protein synthesis. Cell. 1977 May;11(1):187–200. doi: 10.1016/0092-8674(77)90330-0. [DOI] [PubMed] [Google Scholar]
- Friedman R. L., Manly S. P., McMahon M., Kerr I. M., Stark G. R. Transcriptional and posttranscriptional regulation of interferon-induced gene expression in human cells. Cell. 1984 Oct;38(3):745–755. doi: 10.1016/0092-8674(84)90270-8. [DOI] [PubMed] [Google Scholar]
- Galabru J., Hovanessian A. Autophosphorylation of the protein kinase dependent on double-stranded RNA. J Biol Chem. 1987 Nov 15;262(32):15538–15544. [PubMed] [Google Scholar]
- Goldstein J. L., Beaudet A. L., Caskey C. T. Peptide chain termination with mammalian release factor. Proc Natl Acad Sci U S A. 1970 Sep;67(1):99–106. doi: 10.1073/pnas.67.1.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golgher R. R., Williams B. R., Gilbert C. S., Brown R. E., Kerr I. M. Protein kinase activity and the natural occurrence of 2-5A in interferon-treated EMC virus-infected L-cells. Ann N Y Acad Sci. 1980;350:448–458. doi: 10.1111/j.1749-6632.1980.tb20648.x. [DOI] [PubMed] [Google Scholar]
- Hanyu N., Kuchino Y., Nishimura S., Beier H. Dramatic events in ciliate evolution: alteration of UAA and UAG termination codons to glutamine codons due to anticodon mutations in two Tetrahymena tRNAs. EMBO J. 1986 Jun;5(6):1307–1311. doi: 10.1002/j.1460-2075.1986.tb04360.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hovanessian A. G. The double stranded RNA-activated protein kinase induced by interferon: dsRNA-PK. J Interferon Res. 1989 Dec;9(6):641–647. doi: 10.1089/jir.1989.9.641. [DOI] [PubMed] [Google Scholar]
- Innanen V. T., Nicholls D. M. Studies on peptidyl transferase in free ribosomes derived from rat liver. Biochim Biophys Acta. 1974 Aug 29;361(2):221–229. doi: 10.1016/0005-2787(74)90349-9. [DOI] [PubMed] [Google Scholar]
- Jacob C. O., van der Meide P. H., McDevitt H. O. In vivo treatment of (NZB X NZW)F1 lupus-like nephritis with monoclonal antibody to gamma interferon. J Exp Med. 1987 Sep 1;166(3):798–803. doi: 10.1084/jem.166.3.798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohase M., Henriksen-DeStefano D., May L. T., Vilcek J., Sehgal P. B. Induction of beta 2-interferon by tumor necrosis factor: a homeostatic mechanism in the control of cell proliferation. Cell. 1986 Jun 6;45(5):659–666. doi: 10.1016/0092-8674(86)90780-4. [DOI] [PubMed] [Google Scholar]
- Konecki D. S., Aune K. C., Tate W., Caskey C. T. Characterization of reticulocyte release factor. J Biol Chem. 1977 Jul 10;252(13):4514–4520. [PubMed] [Google Scholar]
- Kozak M. Regulation of protein synthesis in virus-infected animal cells. Adv Virus Res. 1986;31:229–292. doi: 10.1016/S0065-3527(08)60265-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuchino Y., Beier H., Akita N., Nishimura S. Natural UAG suppressor glutamine tRNA is elevated in mouse cells infected with Moloney murine leukemia virus. Proc Natl Acad Sci U S A. 1987 May;84(9):2668–2672. doi: 10.1073/pnas.84.9.2668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larner A. C., Jonak G., Cheng Y. S., Korant B., Knight E., Darnell J. E., Jr Transcriptional induction of two genes in human cells by beta interferon. Proc Natl Acad Sci U S A. 1984 Nov;81(21):6733–6737. doi: 10.1073/pnas.81.21.6733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee C. C., Craigen W. J., Muzny D. M., Harlow E., Caskey C. T. Cloning and expression of a mammalian peptide chain release factor with sequence similarity to tryptophanyl-tRNA synthetases. Proc Natl Acad Sci U S A. 1990 May;87(9):3508–3512. doi: 10.1073/pnas.87.9.3508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin D., London I. M. Regulation of protein synthesis: activation by double-stranded RNA of a protein kinase that phosphorylates eukaryotic initiation factor 2. Proc Natl Acad Sci U S A. 1978 Mar;75(3):1121–1125. doi: 10.1073/pnas.75.3.1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luster A. D., Unkeless J. C., Ravetch J. V. Gamma-interferon transcriptionally regulates an early-response gene containing homology to platelet proteins. Nature. 1985 Jun 20;315(6021):672–676. doi: 10.1038/315672a0. [DOI] [PubMed] [Google Scholar]
- Melton D. A., Krieg P. A., Rebagliati M. R., Maniatis T., Zinn K., Green M. R. Efficient in vitro synthesis of biologically active RNA and RNA hybridization probes from plasmids containing a bacteriophage SP6 promoter. Nucleic Acids Res. 1984 Sep 25;12(18):7035–7056. doi: 10.1093/nar/12.18.7035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merlin G., Chebath J., Benech P., Metz R., Revel M. Molecular cloning and sequence of partial cDNA for interferon-induced (2'-5')oligo(A) synthetase mRNA from human cells. Proc Natl Acad Sci U S A. 1983 Aug;80(16):4904–4908. doi: 10.1073/pnas.80.16.4904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meurs E., Chong K., Galabru J., Thomas N. S., Kerr I. M., Williams B. R., Hovanessian A. G. Molecular cloning and characterization of the human double-stranded RNA-activated protein kinase induced by interferon. Cell. 1990 Jul 27;62(2):379–390. doi: 10.1016/0092-8674(90)90374-n. [DOI] [PubMed] [Google Scholar]
- Nakamura M., Manser T., Pearson G. D., Daley M. J., Gefter M. L. Effect of IFN-gamma on the immune response in vivo and on gene expression in vitro. 1984 Jan 26-Feb 1Nature. 307(5949):381–382. doi: 10.1038/307381a0. [DOI] [PubMed] [Google Scholar]
- Philipson L., Andersson P., Olshevsky U., Weinberg R., Baltimore D., Gesteland R. Translation of MuLV and MSV RNAs in nuclease-treated reticulocyte extracts: enhancement of the gag-pol polypeptide with yeast suppressor tRNA. Cell. 1978 Jan;13(1):189–199. doi: 10.1016/0092-8674(78)90149-6. [DOI] [PubMed] [Google Scholar]
- Saunders M. E., Gewert D. R., Tugwell M. E., McMahon M., Williams B. R. Human 2-5A synthetase: characterization of a novel cDNA and corresponding gene structure. EMBO J. 1985 Jul;4(7):1761–1768. doi: 10.1002/j.1460-2075.1985.tb03848.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stadtman T. C. Selenium biochemistry. Annu Rev Biochem. 1990;59:111–127. doi: 10.1146/annurev.bi.59.070190.000551. [DOI] [PubMed] [Google Scholar]
- Staeheli P., Haller O., Boll W., Lindenmann J., Weissmann C. Mx protein: constitutive expression in 3T3 cells transformed with cloned Mx cDNA confers selective resistance to influenza virus. Cell. 1986 Jan 17;44(1):147–158. doi: 10.1016/0092-8674(86)90493-9. [DOI] [PubMed] [Google Scholar]
- Tamm I., Lin S. L., Pfeffer L. M., Sehgal P. B. Interferons alpha and beta as cellular regulatory molecules. Interferon. 1987;9:13–74. [PubMed] [Google Scholar]
- Valle R. P., Morch M. D., Haenni A. L. Novel amber suppressor tRNAs of mammalian origin. EMBO J. 1987 Oct;6(10):3049–3055. doi: 10.1002/j.1460-2075.1987.tb02611.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valle R. P., Morch M. D. Stop making sense: or Regulation at the level of termination in eukaryotic protein synthesis. FEBS Lett. 1988 Aug 1;235(1-2):1–15. doi: 10.1016/0014-5793(88)81225-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolpe S. D., Cerami A. Macrophage inflammatory proteins 1 and 2: members of a novel superfamily of cytokines. FASEB J. 1989 Dec;3(14):2565–2573. doi: 10.1096/fasebj.3.14.2687068. [DOI] [PubMed] [Google Scholar]
- Yoshinaka Y., Katoh I., Copeland T. D., Oroszlan S. Murine leukemia virus protease is encoded by the gag-pol gene and is synthesized through suppression of an amber termination codon. Proc Natl Acad Sci U S A. 1985 Mar;82(6):1618–1622. doi: 10.1073/pnas.82.6.1618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zinoni F., Birkmann A., Stadtman T. C., Böck A. Nucleotide sequence and expression of the selenocysteine-containing polypeptide of formate dehydrogenase (formate-hydrogen-lyase-linked) from Escherichia coli. Proc Natl Acad Sci U S A. 1986 Jul;83(13):4650–4654. doi: 10.1073/pnas.83.13.4650. [DOI] [PMC free article] [PubMed] [Google Scholar]