Abstract
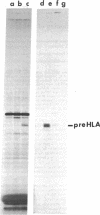
A clone (pHLA-1) containing HLA-specific cDNA was constructed by reverse transcription of partially purified HLA mRNA from the human lymphoblastoid cell line LKT. The identity of pHLA-1 was established by its ability to hybridize to HLA heavy chain mRNA and by nucleotide sequence analysis. The pHLA-1 cDNA insert (approximately 525 base pairs) corresponds to the COOH-terminal 46 amino acids of an HLA-A, -B, or -C antigen (15 residues from the hydrophobic region and the remainder from the COOH-termial hydrophilic region), together with a portion of the 3' untranslated region of the mRNA.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bach F. H., van Rood J. J. The major histocompatibility complex--genetics and biology. (First of three parts). N Engl J Med. 1976 Oct 7;295(15):806–813. doi: 10.1056/NEJM197610072951504. [DOI] [PubMed] [Google Scholar]
- Ballou B., McKean D. J., Freedlender E. F., Smithies O. HLA membrane antigens: sequencing by intrinsic radioactivity. Proc Natl Acad Sci U S A. 1976 Dec;73(12):4487–4491. doi: 10.1073/pnas.73.12.4487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birnboim H. C., Doly J. A rapid alkaline extraction procedure for screening recombinant plasmid DNA. Nucleic Acids Res. 1979 Nov 24;7(6):1513–1523. doi: 10.1093/nar/7.6.1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bridgen J., Snary D., Crumpton M. J. Isolation and N-terminal amino acid sequence of membrane-bound human HLA-A and HLA-B antigens. Nature. 1976 May 20;261(5557):200–205. doi: 10.1038/261200a0. [DOI] [PubMed] [Google Scholar]
- Francke U., Pellegrino M. A. Assignment of the major histocompatibility complex to a region of the short arm of human chromosome 6. Proc Natl Acad Sci U S A. 1977 Mar;74(3):1147–1151. doi: 10.1073/pnas.74.3.1147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman D. W., Pober J. S., White J., Bayley H. Selective labelling of the hydrophobic segments of intrinsic membrane proteins with a lipophilic photogenerated carbene. Nature. 1979 Aug 30;280(5725):841–843. doi: 10.1038/280841a0. [DOI] [PubMed] [Google Scholar]
- Kafatos F. C., Jones C. W., Efstratiadis A. Determination of nucleic acid sequence homologies and relative concentrations by a dot hybridization procedure. Nucleic Acids Res. 1979 Nov 24;7(6):1541–1552. doi: 10.1093/nar/7.6.1541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katcoff D., Nudel U., Zevin-Sonkin D., Carmon Y., Shani M., Lehrach H., Frischauf A. M., Yaffe D. Construction of recombinant plasmids containing rat muscle actin and myosin light chain DNA sequences. Proc Natl Acad Sci U S A. 1980 Feb;77(2):960–964. doi: 10.1073/pnas.77.2.960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein J. The major histocompatibility complex of the mouse. Science. 1979 Feb 9;203(4380):516–521. doi: 10.1126/science.104386. [DOI] [PubMed] [Google Scholar]
- Krangel M. S., Orr H. T., Strominger J. L. Assembly and maturation of HLA-A and HLA-B antigens in vivo. Cell. 1979 Dec;18(4):979–991. doi: 10.1016/0092-8674(79)90210-1. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. Sequencing end-labeled DNA with base-specific chemical cleavages. Methods Enzymol. 1980;65(1):499–560. doi: 10.1016/s0076-6879(80)65059-9. [DOI] [PubMed] [Google Scholar]
- Nelson T., Brutlag D. Addition of homopolymers to the 3'-ends of duplex DNA with terminal transferase. Methods Enzymol. 1979;68:41–50. doi: 10.1016/0076-6879(79)68005-9. [DOI] [PubMed] [Google Scholar]
- Olson K., Harvey C. Determination of the 3' terminal nucleotide of DNA fragments. Nucleic Acids Res. 1975 Mar;2(3):319–325. doi: 10.1093/nar/2.3.319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orr H. T., Lancet D., Robb R. J., Lopez de Castro J. A., Strominger J. L. The heavy chain of human histocompatibility antigen HLA-B7 contains an immunoglobulin-like region. Nature. 1979 Nov 15;282(5736):266–270. doi: 10.1038/282266a0. [DOI] [PubMed] [Google Scholar]
- Orr H. T., Lopez de Castro J. A., Parham P., Ploegh H. L., Strominger J. L. Comparison of amino acid sequences of two human histocompatibility antigens, HLA-A2 and HLA-B7: location of putative alloantigenic sites. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4395–4399. doi: 10.1073/pnas.76.9.4395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orr H. T., López de Castro J. A., Lancet D., Strominger J. L. Complete amino acid sequence of a papain-solubilized human histocompatibility antigen, HLA-B7. 2. Sequence determination and search for homologies. Biochemistry. 1979 Dec 11;18(25):5711–5720. doi: 10.1021/bi00592a030. [DOI] [PubMed] [Google Scholar]
- Ploegh H. L., Cannon L. E., Strominger J. L. Cell-free translation of the mRNAs for the heavy and light chains of HLA-A and HLA-B antigens. Proc Natl Acad Sci U S A. 1979 May;76(5):2273–2277. doi: 10.1073/pnas.76.5.2273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Proudfoot N. J., Brownlee G. G. 3' non-coding region sequences in eukaryotic messenger RNA. Nature. 1976 Sep 16;263(5574):211–214. doi: 10.1038/263211a0. [DOI] [PubMed] [Google Scholar]
- Ricciardi R. P., Miller J. S., Roberts B. E. Purification and mapping of specific mRNAs by hybridization-selection and cell-free translation. Proc Natl Acad Sci U S A. 1979 Oct;76(10):4927–4931. doi: 10.1073/pnas.76.10.4927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robb R. J., Terhorst C., Strominger J. L. Sequence of the COOH-terminal hydrophilic region of histocompatibility antigens HLA-A2 and HLA-B7. J Biol Chem. 1978 Aug 10;253(15):5319–5324. [PubMed] [Google Scholar]
- Roberts T. M., Lauer G. D. Maximizing gene expression on a plasmid using recombination in vitro. Methods Enzymol. 1979;68:473–482. doi: 10.1016/0076-6879(79)68036-9. [DOI] [PubMed] [Google Scholar]
- Springer T. A., Strominger J. L. Detergent-soluble HLA antigens contain a hydrophilic region at the COOH-terminus and a penultimate hydrophobic region. Proc Natl Acad Sci U S A. 1976 Jul;73(7):2481–2485. doi: 10.1073/pnas.73.7.2481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tu C. P., Cohen S. N. 3'-end labeling of DNA with [alpha-32P]cordycepin-5'-triphosphate. Gene. 1980 Jul;10(2):177–183. doi: 10.1016/0378-1119(80)90135-3. [DOI] [PubMed] [Google Scholar]
- Wickens M. P., Buell G. N., Schimke R. T. Synthesis of double-stranded DNA complementary to lysozyme, ovomucoid, and ovalbumin mRNAs. Optimization for full length second strand synthesis by Escherichia coli DNA polymerase I. J Biol Chem. 1978 Apr 10;253(7):2483–2495. [PubMed] [Google Scholar]
- Wieringa B., Mulder J., van der Ende A., Bruggeman A., Ab G., Gruber M. Purification of vitellogenin mRNA and serum albumin mRNA from avian liver by preparative gel electrophoresis. Eur J Biochem. 1978 Aug 15;89(1):67–79. doi: 10.1111/j.1432-1033.1978.tb20897.x. [DOI] [PubMed] [Google Scholar]